Standard Operating Procedure for AQL and Sampling of Packaging Materials (PM) in pharmaceuticals
Standard Operating Procedure for AQL and Sampling of Packaging Materials (PM) in pharmaceuticals
AQL and Sampling of Packaging Materials in Pharmaceuticals as per ISO 2859-1, ANSI/ASQC Z1.4, ASTM E2234, NF06-022, BS 6001, DIN 40080, and US Military Standards (MIL STD – 105E).
Refer to Online AQL Calculator to understand the sampling plan and corresponding Acceptance Quality Level.
1. Purpose:
To provide a standard operating procedure for Sampling of Packaging Materials (PM) in pharmaceuticals.
2. Scope
The scope of this SOP is applicable for Sampling of Packaging Materials (PM) in pharmaceuticals at [company name].
3. Responsibility
Quality Control Analyst:
Sampling of Packaging Materials
Quality Control Packing Material Section Head:
Monitoring the activity and review the records
Quality Control Laboratory Head:
To ensure compliance to the procedure
4. Definitions
Not applicable
5. Procedure
• On receipt of Packing Material in the warehouse, the warehouse enters details of received material in the material management software. As soon as the Goods Inward Note (GRN) is prepared, the Laboratory Information Management System (LIMS) database for Packing Material awaiting sampling is updated.
• Daily, Quality Control laboratory personnel responsible for the packing material section (Packing Material section head) will check the list of material populated in the system displays as material to be tested.
• Packing material section head will also inquire about the priority of the manufacturing department before planning Packing Material Testing or Refer Monthly Production Planning Schedule.
• Packing material section head will allocate the material to be sampled to the Quality Control Laboratory Person responsible for sampling Packing Material (Sampler) in the LIMS.
• The sampler will log in to the LIMS and print the Packing Material sampling worksheet.
• The sampler, will reach the warehouse department and request warehouse staff to take the material to be sampled at the sampling area.
• The sampler shall verify the cleanliness of the sampling area before bringing the material inside the sampling area and starting the sampling activity.
• On arrival of Packing Material inside the sampling area, sampler will verify the material label, manufacturers’ batch number, control number/ A.R. No./ unique identification number of material (generated by material management software of the organization), and ensure that material arrived in the room is the same material that is supposed to be sampled as per sampling worksheet.
• The sampler will also ensure that the Packing Material is free from external damages. If the any damage noted, it shall be marked separately and informed to the warehouse person, packing material section head, and Quality Assurance Person.
• The sampler will verify the Packing Material using a sampling worksheet to ensure the following:
• Packing Materials shall have the label as “Quarantine” to each container with details of Material Name, Material Code, Control No./ A.R. No., Material Manufacturer’s name/ Vendor name, Manufacturer Batch No., Manufacturing Date, Expiry Date, Quantity of material received, Number of containers received, Pack Size, Warehouse document serial number, and Total number of Containers..
• Materials shall not be accepted when the material name is not listed in the approved vendor list, if abnormality is observed during sampling, or Certificate of Analysis (CoA) not received with the material.
• The packing material section head shall verify the CoA received from the material manufacturer and compare it against the specification implemented in the organization. The packing material section head will ensure that the analysis results of the material manufacturer are within the organization’s specification limit. In case of any deviation noted, it shall be informed to the Quality Control laboratory head and Quality Assurance head to take a call on whether material shall be accepted or rejected.
• Before starting the sampling, ensure that the Sampling area environmental condition, such as Temperature, Relative Humidity, and Differential Air Pressure, is within the defined and acceptable range.
• Only one material shall be sampled at a time in Sampling Area.
• For sampling of primary packing material, ensure that Reverse Laminar Airflow (RLAF) is switched “ON” at least before [specify the time which is established through recovery study, it vary from 10 minutes to half an hour]. Record the pressure differential across the pre-filter, intermediate, and HEPA filter and ensure that readings are within the defined range. In case of any abnormality, sampling shall not be started. Instead, inform to the engineering department to rectify before beginning the sampling activity.
• Containers to be sampled shall be taken within the safe zone of RLAF. A sampling of volatile materials shall be done at solvent sampling and dispensing area.
• Before starting the sampling of material, the sampler shall follow safety precautions. Sampler needs to use required Personal Protective Equipment (PPE) during sampling. Example, Nose Mask, Hand Gloves, Safety Goggles, etc.
• Material Safety Data Sheet [MSDS] should be handy in the area as applicable.
• Before starting the sampling activity, make an entry in the logbook for sampling activity, such as material name, control number, RLAF start time (in case of primary packing material), and sampling start time.
• Once the sampling activity is completed, enter the sampling end time.
• Sampling of Secondary Packaging Materials and Tertiary Packaging Material shall be done at sampling area for respective material.
• In case of sterile packing material (Pre-sterilized packing material) satellite sample will be received along with the material. This satellite sample shall be collected. Ensure that integrity of sample container/ sample bag is maintained. In case does not provides satellite pack, any one entire container/ pack shall be collected as sample.
• In case of sterile material, verify each container for evidence of sterilization with the help of indicators available on each pack. Example, indicator for Gamma Radiation Sterilization or indicator for Ethylene Oxide (EtO) sterilization.
• Entire sterile packing material sample shall be sent to microbiologist department to carryout sterility testing. The remaining samples shall be handed over to Quality Control analyst for visual inspection check, Physical and Chemical Tests.
• Leaflets/ Patient Information Leaflet (inserts or Outserts) visual inspection to be performed in the Quality Control Laboratory.
• For Aluminum/ paper Foil, PVC, PVdC Film, before carryout sampling discard about 1/2 meter material from role.
• If number of Joints is more than three, it shall not be accepted and information for number of joints shall be recorded in packing material sampling and observation worksheet.
• Sampling quantity to be withdrawn shall be equal from all the selected number of containers to be sampled.
• For printed packing material, multiple ups sheet/ Uncut Printed Sheet shall be provided by the packing material manufacturer with the material received and it must be approved by material manufacturer. This shall help to verify the correctness of Text matter and Appearance.
• Examples of printed packing materials are Labels, Cartons, Catch Covers, Patient Information Leaflet, Literatures, Printed Foils, etc.
• After completion of sampling, sampler shall close the container or pack to ensure material storage adequately.
• Personal Protective Equipment (PPE) such as Nose Mask, Hand Gloves and Safety Goggles, shall be used during sampling.
• Samples quantity for the sample to be collected shall be as per sampling worksheet.
• For visual inspection of packing material (spot check of packing material in warehouse during sampling), and sampling quantity is defined based on ISO 2859-1 standard. It is an international standard with equivalents in all national regulations (ANSI/ASQC Z1.4, ASTM E2234, NF06-022, BS 6001, DIN 40080) and US Military Standards (MIL STD – 105E).
• In this procedure, sample size is considered based on ISO 2859 with the following criteria:
(i) Number of packs or containers to be opened for visual inspection and sampling shall be decided as per Table 1. (General inspection level III is considered).
(ii) Acceptance and rejection criteria for visual inspection Primary Packing Material shall be decided as per Table 2. (General inspection level III is considered).
(iii) Acceptance and rejection criteria for visual inspection Secondary and Tertiary Packing Material shall be decided as per Table 3. (General inspection level II is considered).
Table 1: Number of packs or containers of Packing Material to be opened for visual inspection and sampling (General inspection level III is considered)
| Sample size Code | Total number of packs or containers received | Total number of packs or containers to be opened for visual inspection and collection of sample for analysis |
| B | 02 – 08 | 3 |
| C | 09 – 15 | 5 |
| D | 16 – 25 | 8 |
| E | 26 – 50 | 13 |
| F | 51 – 90 | 20 |
| G | 91 – 150 | 32 |
| H | 151 – 280 | 50 |
| J | 281 – 500 | 80 |
| K | 501 – 1200 | 125 |
Note: Sample quantity for analysis will be derived from method of analysis for respective material. Sample from each container = Total sample quantity/ Total number of packs or containers to be opened as per Table 1
Reference: Table as per ISO 2859-1
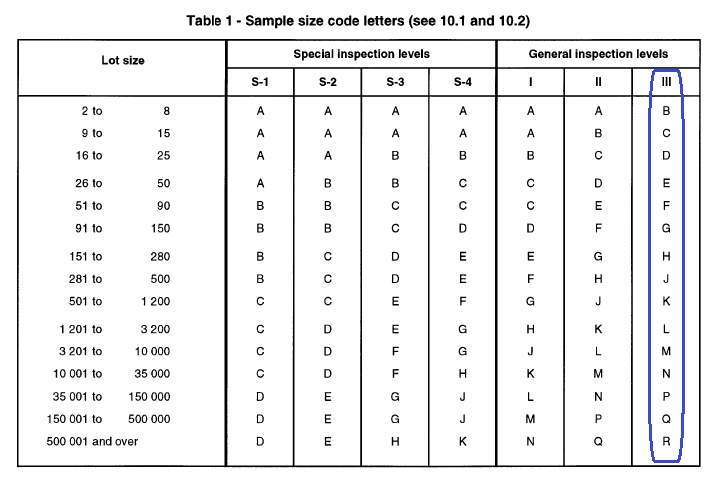
Table 2: Acceptance and rejection criteria for visual inspection of Primary Packing Material
| Lot Size (Total number of items received in entire consignment) | Sample size code | Sample Size (Total samples collected for visual inspection) | Critical Ac | Critical Re | Major Ac | Major Re | Minor Ac | Minor Re |
| 2 to 8 | B | 3 | 0 | 1 | 0 | 1 | 0 | 1 |
| 9 to 15 | C | 5 | 0 | 1 | 0 | 1 | 0 | 1 |
| 16 to25 | D | 8 | 0 | 1 | 0 | 1 | 0 | 1 |
| 26 to 50 | E | 13 | 0 | 1 | 0 | 1 | 1 | 2 |
| 51 to 90 | F | 20 | 0 | 1 | 0 | 1 | 1 | 2 |
| 91 to 150 | G | 32 | 0 | 1 | 1 | 2 | 2 | 3 |
| 151 to 280 | H | 50 | 0 | 1 | 1 | 2 | 3 | 4 |
| 281 to 500 | J | 80 | 0 | 1 | 2 | 3 | 5 | 6 |
| 501 to 1,200 | K | 125 | 0 | 1 | 3 | 4 | 7 | 8 |
| 1,201 to 3,200 | L | 200 | 0 | 1 | 5 | 6 | 10 | 11 |
| 3,201 to 10,000 | M | 315 | 0 | 1 | 7 | 8 | 14 | 15 |
| 10,001 to 35,000 | N | 500 | 0 | 1 | 10 | 11 | 21 | 22 |
| 35,001 to 150,000 | P | 800 | 0 | 1 | 14 | 15 | 21 | 22 |
| 150,001 to 500,000 | Q | 1250 | 0 | 1 | 21 | 22 | 21 | 22 |
| 500,001 and over | R | 2000 | 0 | 1 | 21 | 22 | 21 | 22 |
Note:
(i) Ac – Acceptance number, Re – Rejection number
(ii) General inspection level III is considered
(iii) Acceptance Criteria as per Normal Inspection plan – Critical at 0 %, Major at 1.0 %, and Minor at 2.5 %.
Table 3: Acceptance and rejection criteria for visual inspection of Secondary and Tertiary Packing Material
| Lot Size (Total number of items received in entire consignment) | Sample size code | Sample Size (Total samples collected for visual inspection) | Critical Ac | Critical Re | Major Ac | Major Re | Minor Ac | Minor Re |
| 2 to 8 | A | 2 | 0 | 1 | 0 | 1 | 0 | 1 |
| 9 to 15 | B | 3 | 0 | 1 | 0 | 1 | 0 | 1 |
| 16 to 25 | C | 5 | 0 | 1 | 0 | 1 | 0 | 1 |
| 26 to 50 | D | 8 | 0 | 1 | 0 | 1 | 0 | 1 |
| 51 to 90 | E | 13 | 0 | 1 | 0 | 1 | 1 | 2 |
| 91 to 150 | F | 20 | 0 | 1 | 0 | 1 | 1 | 2 |
| 151 to 280 | G | 32 | 0 | 1 | 1 | 2 | 2 | 3 |
| 281 to 500 | H | 50 | 0 | 1 | 1 | 2 | 3 | 4 |
| 501 to 1,200 | J | 80 | 0 | 1 | 2 | 3 | 5 | 6 |
| 1,201 to 3,200 | K | 125 | 0 | 1 | 3 | 4 | 7 | 8 |
| 3,201 to 10,000 | L | 200 | 0 | 1 | 5 | 6 | 10 | 11 |
| 10,001 to 35,000 | M | 315 | 0 | 1 | 7 | 8 | 14 | 15 |
| 35,001 to 150,000 | N | 500 | 0 | 1 | 10 | 11 | 21 | 22 |
| 150,001 to 500,000 | P | 800 | 0 | 1 | 14 | 15 | 21 | 22 |
| 500,001 and over | Q | 1250 | 0 | 1 | 21 | 22 | 21 | 22 |
Note:
(i) Ac – Acceptance number, Re – Rejection number
(ii) General inspection level II is considered
(iii) Acceptance Criteria as per Normal Inspection plan – Critical at 0 %, Major at 1.0 %, and Minor at 2.5 %.
• Sampling person needs to record the visual inspection observation in the “Packing material sampling and observation worksheet”.
• Acceptance or rejection of packing material is decided based on the Table 2 for Primary Packing Material and Table 3 for Secondary and Tertiary Packing Material.
• Defects are classified as Critical, Major and Minor based on the following definitions:
Critical Defects: The defect in the material has highest potential to make pharmaceutical formulation unsafe with respect to product quality and patient safety.
Major Defects: The defect in the material that may impact the packaging operation and generates defects during packing operation and have potential for multiple customer complaints but does not have patient safety risk.
Minor Defects: The defect in the material that may impact on product appearance and have potential for few customer complaints, but may not have impact on product quality and patient safety.
• In case the samples are received as satellite pack for testing, and quantity of sample is insufficient to carryout AQL, AQL can be done on intact sample pack/ container through the transparent bag without opening the pack. After completion of AQL, pack shall be return to warehouse.
• AQL inspection to be done while new lot of material received. During re-test AQL inspection shall not be carried out.
• Once the sampling is completed, sampler shall affix “Under Test” Labels to all the packs. Under test label shall have details Material Name, Material Code, A.R. No. / Control No., Manufacturer name, Manufacturer Batch No., Manufacturing Date, Expiry Date, quantity received, Number of pack/ container, Pack Size, Sampled By, Sampled on, Sampled Pack Nos.
• Number of “Under Test” label generated shall be total number of packs + One. One additional label shall be generated to affix with the analytical report.
• Details of sampling and visual inspection details shall be recorded in the “Packing material sampling and observation worksheet”.
• Sampler shall carry required tools such as polybag, Scissors, and Rubber Band etc.
• When sampling is planned, QC sampler will inform to the warehouse person to locate the material and take it to the sampling area.
•Identify the number of packs to be open for AQL and sampling as per Table 1.
• Ensure that the packs are clean. If require, packs shall be cleaned from external surface.
• Note the quantity of material to be sampled. The sample quantity is calculated using method of analysis and it is listed in the “List Packing Material Sample Quantity”.
• Number of pack to be sampled shall be derived using Table 2 and Table 3 for primary packing material, and secondary and tertiary packing material respectively.
• Record the AQL results and quantity of material sampled in the “Packing material sampling and observation worksheet”.
• Example of deriving packs to be opened and samples to be collected from number of containers.
| Total number of packs for HDPE containers received (Primary Packing Material | 20 Packs |
| Total number of HDP bottles received | 20000 Bottles |
| Each pack consist of number of bottles | 1000 Bottles |
| Based on the Table 1, Sample Size Code is D. Hence, total number of packs or containers to be opened for visual inspection and collection of sample for analysis | 8 Packs |
| Total sample quantity required for analysis to carryout full testing as per method of analysis are | 200 bottles |
| Sample to be collected from each pack (from 8 packs) are 200 divided by 8 | 25 bottles from each container |
| Packs to be considered for sampling will be representative of entire lot received | Pack 1, 3, 6, 9, [10 or 11], 12, 15, 18, and 20 |
| Based on the Table 2, Sample Size Code is N. Hence, total number of bottles to be collected for AQL/ Visual inspection are | 500 |
| Sample to be collected from each pack [from 8 packs (Pack 1, 3, 6, 9, [10 or 11], 12, 15, 18, and 20)] are | 62 from 4 packs and 63 from 4 packs container |
| Critical defect Acceptance | 0 |
| Critical defect Rejection | 1 |
| Major defect Acceptance | 10 |
| Major defect Rejection | 11 |
| Minor defect Acceptance | 21 |
| Minor defect Rejection | 22 |
• The container which is sampled will be affixed with label “Sampled” for identification purpose.
• “Under Test” label shall be affixed at inner core in case of aluminum foils, PVC or PCV/PVdC Films, or roll labels.
• Once the sampling is completed, request to warehouse person to transfer the material to its respective location.
• Sampler shall send the sample to Quality Control laboratory for analysis and details shall be recorded in packing material inward register.
• Sample shall be stored at location as per required storage condition.
6. Frequency
For all new incoming packing material and during retest of material.
7. Formats
7.1 Packing material sampling and observation worksheet
| Description | Observation/ Compliance |
| Material Name | |
| Material Code | |
| A.R. No./ Control No. | |
| Material Manufacturer | |
| manufacturing Batch No. | |
| Manufacturing Date | |
| Expiry Date | |
| Quantity received | |
| Number of Packs received | |
| Pack Size | |
| Type of Packaging | |
| Quantity Sampled | |
| Sampled Pack No. | |
| Foreign Matter/ Contamination | |
| Packaging Condition/ Damage | |
| Sampling Room environmental condition: Differential Pressure l Readings, Temperature and Relative Humidity | |
| Manufacturer’s CoA compliance with organizations specification | |
| Material is pre-sterilized (Yes/ No) | |
| Mode of sterilization (Gamma/ ETO/ Other specify __________) | |
| Sterilization indicator availability on each container (Yes/ No) | |
| Indicator color observed [Specify standard color for each type of sterilization for sampler’s understanding and observation] | |
| Number of Joints (Applicable for foil and film) | |
| Total number of packs received | |
| Total number of packing material quantity received | |
| Each pack consist of number of packing material | |
| Sample Size Code as per Table 1 for total number of packs or containers to be opened for visual inspection and collection of sample for analysis | |
| Total sample quantity required for analysis to carryout full testing as per method of analysis are | |
| Sample to be collected from each pack | |
| Pack Nos. to be considered for sampling | |
| Sample Size Code as per Table 2 (Primary Packing Material) or Table 3 (Secondary and Tertiary Packing Material) for total number of packing material to be collected for AQL/ Visual inspection are | |
| Sample to be collected from each pack for visual inspection/ AQL are | |
| Critical defect Acceptance | |
| Critical defect Rejection | |
| Critical defect observed | |
| Major defect Acceptance | |
| Major defect Rejection | |
| Major defect observed | |
| Minor defect Acceptance | |
| Minor defect Rejection | |
| Minor defect observed | |
| Visual inspection/ AQL Complies/ Does Not Comply | |
| Storage condition | |
| Material sampled and AQL done By | |
| Checked by |
7.2 Packing material sample inward register (Content of logbook)
Sr. No.
Sampling Date
Material Name
A.R. No./ Control No.
Quantity Received
Quantity Sampled
Manufacturer name
Material inward entry made by
Date of material approved/ rejected
Analyst name
Analyst sign
Leftover sample quantity after analysis
Leftover sample quantity destroyed by
7.3 List Packing Material Sample Quantity (Content of logbook)
Sr. No.
Name of Material
Quantity to be sampled for newly received material
Quantities to be sampled during sampling for re-test
7.4 Packing Material defect classification for AQL/ Visual inspection
| Material Name | Printed Carton |
| Critical defects | Mix-ups, Missing text matter or color, Missing embossing, Wet cartons, Dirty cartons |
| Major defects | Locking defects, Improper pasting, Stuck cartons, Smudging of printing matter, Shifting of text matters |
| Minor defects | Improper creasing or folding, Shade/ color variation |
| Material Name | Plain Carton |
| Critical defects | Mix-ups, Wet cartons, Dirty cartons |
| Major defects | Locking defects, Improper pasting, Stuck cartons |
| Minor defects | Improper creasing or folding, Shade/ color variation |
| Material Name | Catch Covers |
| Critical defects | Mix-ups, Missing text matter or color, Missing embossing, Wet Catch Covers, Dirty Catch Covers |
| Major defects | Locking defects, Improper pasting, Stuck Catch Covers, Smudging of printing matter, Shifting of text matters |
| Minor defects | Improper creasing or folding, Shade/ color variation |
| Material Name | Labels |
| Critical defects | Mix-ups, Missing text matter or color, Wet Labels, Dirty Labels |
| Major defects | Improper pasting, Stuck Labels, Smudging of printing matter, Shifting of text matters, Improper Cutting, Torn Labels |
| Minor defects | Shade/ color variation |
| Material Name | Inserts, Outserts, Leaflets, PIL, Booklet |
| Critical defects | Mix-ups, Missing text matter or color, Wet Labels, Dirty material |
| Major defects | Improper Cutting, Smudging of printing matter, Torn Leaflets, Shifting of text Matters |
| Minor defects | Shade/ color variation |
| Material Name | HDPE Drum / Fiber Drum |
| Critical defects | Extraneous dust or particulate matter, Hole, Cut |
| Major defects | Damaged container, Molding Defects, De-shape |
| Minor defects | Shade/ color variation |
| Material Name | Plain Alu Foil, PVC Film, PVC Acler, PVC-PVdC Film, Alu Ali Foil, Peelable foil |
| Critical defects | Mix-ups, Damaged core |
| Major defects | Loose winding, Telescopic winding, Stain |
| Minor defects | Shade/ color variation, Joints more than accepted numbers |
| Material Name | Printed aluminum foil/ Peelable paper foil |
| Critical defects | Mix-ups, Damaged core, text matter or color missing, Damaged core |
| Major defects | Loose winding, Telescopic winding, Stain |
| Minor defects | Shade/ color variation, Joints more than accepted numbers |
| Material Name | Stretch Film |
| Critical defects | Not applicable |
| Major defects | Wet or dirty |
| Minor defects | Damaged core, Loose winding |
| Material Name | Cotton, Polyester, Rayon |
| Critical defects | Wet or dirty, undesirable odor |
| Major defects | Stain, Foreign particles |
| Minor defects | Not applicable |
| Material Name | Container/ bottle |
| Critical defects | Mix-ups, extraneous dust, foreign/ particulate matter Hole or cut |
| Major defects | Damaged Container, Molding defects, De-shape |
| Minor defects | Shade/ color variation, Stain |
| Material Name | HDPE bottle, LDPE bottle, Container, Closure |
| Critical defects | Mixing of container other than mentioned product Extraneous dust or particulate matter Hole or cut |
| Major defects | Damaged container Moldings defects (De shape) |
| Minor defects | Color Shade Variation |
| Material Name | Rubber Stoppers, Stopper Plunger |
| Critical defects | Damage, cut, Mix-ups |
| Major defects | Molding defects, De-shape |
| Minor defects | Not applicable |
| Material Name | BOPP tape, Glue tape |
| Critical defects | Not applicable |
| Major defects | Shade variation |
| Minor defects | Damaged core |
| Material Name | Printed BOPP tape, Glue tape |
| Critical defects | Not applicable |
| Major defects | Shade variation |
| Minor defects | Damaged core |
| Material Name | Shipper, Corrugated box, Grey board box, Thermocole Box |
| Critical defects | Wet, Dirty |
| Major defects | Stains, Rusted stales, Rust marks, Spots, Damaged, Closing defects, Improper box formation, Improper folding |
| Minor defects | Shade variation |
| Material Name | Air bubble bag |
| Critical defects | Not applicable |
| Major defects | Hole, Cut, Wet, Dirty |
| Minor defects | Damaged bag, Missing air bubbles |
| Material Name | Aluminum Bag, Polyethylene bag , Heat shrink cover |
| Critical defects | Hole, Cut, Wet, Dirty |
| Major defects | Improper sealing, Damaged |
| Minor defects | Not applicable |
| Material Name | Stickers |
| Critical defects | Not applicable |
| Major defects | Hole, Cut, Wet, Dirty |
| Minor defects | Improper pasting ability |
| Material Name | Silica gel bag, Silica gel sachet, Silica gel canister, Molecular sieve sachet, Oxygen absorber canister |
| Critical defects | Hole, Cut, Wet, Dirty, Leak |
| Major defects | Damaged, Improper sealing |
| Minor defects | Not applicable |
| Material Name | Plastic seal (Plain, Printed) |
| Critical defects | Mix-up |
| Major defects | Inappropriate sealing ability |
| Minor defects | Shade variation |
| Material Name | Vials, Pre Filled Syringe, LDPE bottle |
| Critical defects | Extraneous dust or particulate matter, Wet, Dirty |
| Major defects | Cracks, Air bubble, Molding defects, De-shape, Damaged |
| Minor defects | Not applicable |
| Material Name | HDPE/LDPE Nozzles(Eye Dropper) |
| Critical defects | Mix-up, Extraneous dust or particulate matter, Wet, Dirty, Hole, Cut |
| Major defects | Damaged nozzles, Molding defects, De-shape |
| Minor defects | Shade variation |
| Material Name | Aluminum Flip Off Seal |
| Critical defects | Not applicable |
| Major defects | Molding defects, De-shape, Damaged |
| Minor defects | Shade variation |
| Material Name | Plastic Tray |
| Critical defects | Wet, Dirty |
| Major defects | Molding defects, De-shape, Damaged, Cracks |
| Minor defects | Shade variation |
| Material Name | Sterile needles in pack |
| Critical defects | Open Pack |
| Major defects | Not applicable |
| Minor defects | Not applicable |
| Material Name | Sterile syringe in pack |
| Critical defects | Open pack, Hole, Cut |
| Major defects | Damage, Molding defect, De-shape |
| Minor defects | Not applicable |
| Material Name | Plunger Rod |
| Critical defects | Mix-up |
| Major defects | Molding defect, De-shape |
| Minor defects | Not applicable |
| Material Name | Wooden Pallet |
| Critical defects | Wet, Dirty |
| Major defects | Damaged, Stains, Rusted nails, Rust marks, Dust, Any other contamination |
| Minor defects | Not applicable |
| Material Name | Pre-filled Syringe safety guard |
| Critical defects | Broken, Cracked |
| Major defects | Improper spring |
| Minor defects | Spots, Dust |


