Standard Operating Procedure for Dispensing of Raw Materials in warehouse

1. Purpose
To provide a standard operating procedure for Dispensing Raw Materials in the warehouse.
2. Scope
The scope of this SOP is applicable for Dispensing of Raw Materials in the warehouse at [company name]. Solvent dispensing is not covered under this SOP. Dispensing of material for high potent/ high hazardous products is not covered in this SOP.
3. Responsibility
Warehouse person: Dispensing of raw materials
Warehouse supervisor: To verify dispensed material details, to record the activities in the batch manufacturing record
Manufacturing supervisor: To verify dispensed material details against the standard quantity mentioned in batch manufacturing record, to ensure calculation correctness
QA Person: To accord line clearance
Warehouse Head: To monitor the procedure compliance
4. Definitions
Not applicable
5. Procedure
5.1. Manufacturing person shall initiate a material requisition note for the required product. The requisition will contain details of material name, material code, batch number, and required date.
5.2 Warehouse person shall receive the material requisition note and plan for the material dispensing activity as per material requisition.
5.3 Bill Of Material (BOM) with Raw Material details (A.R. Number/ Control No.) for the batch to be dispensed shall be provided to the warehouse by the manufacturing department.
5.4 Material management software shall generate the formulation order where material details shall get arranged automatically based on FEFO (First Expiry First Out) followed by FIFO (First In First Out). Only approved material will get populated in the list.
5.5 Warehouse person shall collect the material from the warehouse storage area as per the list of material and mentioned in the BOM.
5.6 At a time, single batch material shall be dispensed. An open container of the raw materials shall be used first for dispensing.
5.7 Ensure that all required raw materials are in the approved state before dispensing and no material is due for a retest.
5.8 Approved raw material containers shall be collected and transferred into the staging area (through material movement route) after wiping the containers with a lint-free duster. Material shall be kept on the pallet with adequate segregation.
5.9 Warehouse and Manufacturing personnel shall enter into the dispensing area from the man movement change room.
5.10 Warehouse person shall ensure that area is cleaned for dispensing activity before the start of the activity. Accessories used for the dispensing is available with clean status label and instruments such as weighing balance are calibrated.
5.11 Use required Personal protective equipment (PPE) such as hand gloves, nose mask, Earmuff, Earplug, Goggle while working in dispensing area.
5.12 Switched “ON” the Reverse Laminar Air Flow (RLAF) and allow the area to stabilize for the time (Established time studies during recovery study).
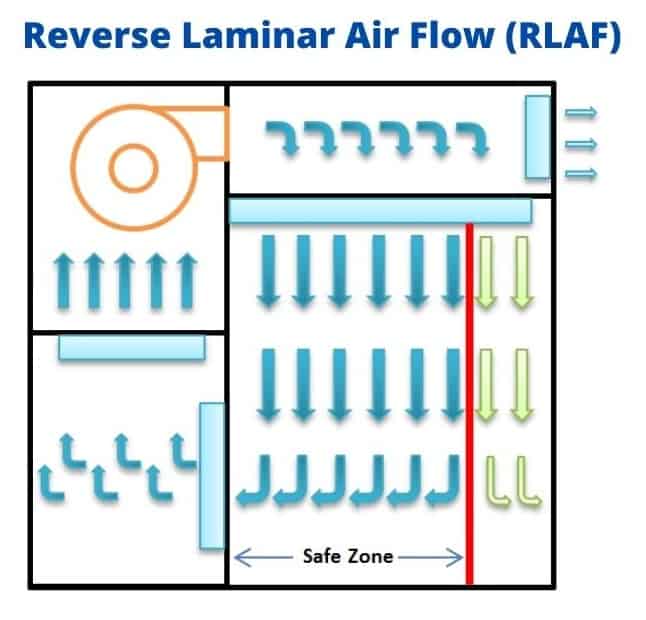
5.13 Ensure that differential pressure reading on Magnehelic gauge is within the allowable limit for primary, secondary, and HEPA filters.
5.14 Ensure that the temperature, relative humidity, and differential pressure are within the acceptable range.
5.15 Before starting the dispensing activity, line clearance should be done to ensure that area is cleaned, no previous product documents, and the residue is available in the area. Line clearance checks for dispensing shall be done by the user department, followed by the Q.A. department. Line clearance shall be recorded in the batch manufacturing record.
5.16 The dispensing activity shall be carried out under the supervision of personnel from the warehouse and manufacturing department.
5.17 The Magnehelic gauge readings of RLAF shall be recorded in the dispensing log. If Magnehelic gauge readings are out of the limit, dispensing shall not be started without rectification.
5.18 Material shall be dispensed under the defined safe zone under RLAF, and a person should stay outside the safe zone. (Safe zone is an area under RLAF where the reverse laminarity of air is maintained).
5.19 The person shall maintain the proper distance between himself and the material to avoid chances of material contamination and himself exposed to the material.
5.20 The dispensing of ‘Active’ and ‘Excipient ingredients shall be carried out in the separate RLAF.
5.21 Excipient shall be dispensed first, followed by active ingredient. The color shall be dispensed at the end.
5.22 During dispensing, details to be verified on the material label, such as Description of the material, Item code number, A.R. Number, Retest date, and expiry date.
5.23 Material shall be weighed as per the quantities defined in BOM using the weighing balance. Ensure that the correct capacity of the weighing balance is used.
5.24 Quantity of the active ingredient shall be calculated by the warehouse based on the formula mentioned in the batch manufacturing record. The calculation shall be verified by the manufacturing and Q.A. person. While calculation, if active potency is more than 100%, it shall be treated as 100%.
5.25 The container in which the material needs to be dispensed shall be kept in the safe zone of RLAF marked. (Safe zone is marked under RLAF)
5.26 Material shall be dispensed using double polybag, and it shall be sealed using re-openable seal (re-openable seal is used to avoid cutting of seal which could be the potential source of contamination because of shredding of seal particle).
5.27 Warehouse supervisor shall verify the standard quantity mentioned in the batch manufacturing record to ensure that the correct amount of material is dispensed.
5.28 The dispensed material shall be labeled with details such as product name, batch number, material name, item code, A.R. Number, Lot No., and container No.
5.29 Manufacturing supervisor shall verify the quantity dispensed against the standard amount, material code, A.R. number, gross weight, tare weight, net weight, and other details on the dispensed material label and verify the correctness concerning the batch manufacturing record.
5.30 Hard gelatin capsules shall be dispensed based on the average weight mentioned in the COA. The required quantity of empty capsules shall be calculated using the average weight of capsule empty capsule.
5.31 Clean utensil shall be used to dispense different materials.
5.32 Active material shall be dispensed using a material dedicated utensil.
5.33. Liquid material shall be dispensed in a stainless steel container.
5.34. Solvent shall be dispensed in a solvent dispensing container (designed to handle solvents).
5.35 “Cleaned” status labels of containers shall be retained at the time of dispensing of raw material and attached with the batch manufacturing record.
5.36 Ensure that the dispensed container wheels shall be cleaned before transferring from dispensing area to the manufacturing room.
5.37 If materials to be dispensed in different lots, each lot shall be segregated to prevent confusion during usage.
5.38 After completing dispensing, the remaining material container shall be transferred to the warehouse through the material airlock.
5.39 Used Utensils and accessories shall be labeled with the status label as “Unclean”.
5.40. At the end of dispensing, if any material physical quantity found different from material management software stock, it should be adjusted in the system up to the allowable amount. If the difference is more than the defined acceptable range, it shall be investigated.
5.41 Before starting of next batch, cleaning of area and RLAF shall be performed.
5.42 Cleaning process shall be documented in the area and equipment logbook.
5.43 In case of environmental conditions found out of limit for temperature, relative humidity, or differential pressure, dispensing activity shall be immediately stopped. Immediately material containers shall be closed. If the condition does not recover in 20 minutes, the material shall be transferred back to the respective storage location. (20 minutes considered based on the response time of the engineering department for rectification).
5.44 After completion of dispensing activity, personnel working in the area shall remove the hand gloves and cut them to prevent re-use of it. Discard the gloves in the waste bin.
5.45 Any spillage of material while handling shall be handled using respective ingredient Material Safety Data Sheet (MSDS).
6. Frequency
Every batch manufacturing
7. Formats
Format for dispensing label
| DISPENSED MATERIAL |
| Ingredient : |
| Item Code : |
| Product : |
| Batch No. : |
| Lot No.: |
| A.R. No.: |
| Tare weight: |
| Net Weight: |
| Gross Weight: |
| Container Number. |
| Done By: |
| Checked By: |
Format for Raw material dispensing and RLAF cleaning logbook
Following content should be covered in the log book
RLAF No.
Date
RLAF Start Time (Hrs.)
Magnehelic gauge pressure reading
Product Name
Batch No.
Batch Size
Dispensing start time (Hrs.)
Material code
Control No.
Dispensed quantity
Dispensing end time (Hrs.)
Dispensed by
Checked By
Cleaning start time
Cleaning end time
Cleaned by
Checked by
RLAF stop time
RLAF stopped by



