Top 70+ Interview Questions for Pharmaceutical Microbiology

This page covers most of the interview questions and answers asked during a technical interview round of Pharmaceutical Microbiology.
You will find interview questions and answers on microbiology basics, pharmacopoeial chapter and sections applicable for microbiology, Culture Media, Growth Promotion test, microbiological quality control for sterile and non-sterile dosage forms, good practice in a microbiological laboratory, aseptic technique to be followed in the microbiological laboratory, pure culture, Pyrogens and endotoxins, different staining techniques used in the pharmaceutical microbiology laboratory, microbial identification, Gram-staining technique, fungal staining, approved culture collections, sterility testing and environmental monitoring.
The interview questions cover questions from basic to advance level of technical aspects. These interview questions and answers will help to crack an interview, enhance your knowledge, and also be helpful for the interviewer who is involved in the recruitment process.
You will find it much more enjoyable while going through these interview questions and answers. So enjoy learning, and best of luck with your interview! Happy Learning.
1. Explain the parts of compound light microscopes used in the pharmaceutical microbiology laboratory?

● Illuminator: Light source at the base of the microscope enable better view of object;
● Condensor: This is a two lens system that collects and concentrates light from the illuminator and directs it to the iris diaphragm;
● Iris diaphragm: regulates the amount of light entering the lens system;
● Stage: This is a platform for the slide with hole in the center to let light from the illuminator pass through. It consists of clips to hold the slide lace;
● Eye pieces: To view object;
● Objectives: To magnify the object as per rating on it
2. What are the pharmacopoeial chapter and sections applicable for microbiology?
| Pharmacopoeial chapter(s) | Use |
| Microbiological examination of nonsterile products: total viable aerobic count (Ph. Eur. 2.6.12, USP <61>) USP <2021> Microbial enumeration tests-nutritional and dietary supplements USP <2023> Microbiological attributes of nonsterile nutritional and dietary supplements | Organism count in raw materials, water, finished products |
| Microbiological examination of nonsterile products: tests for specified organisms (Ph. Eur. 2.6.13, USP <62>) USP <2022> Microbiological procedures for absence of specified microorganismsnutritional and dietary supplements | Type of organisms present in raw materials, water, finished products |
| Ph. Eur. 5.1.4 Microbiological quality of pharmaceutical preparations/USP <1111> Microbiological attributes of nonsterile pharmaceutical products | Determining limits and control factors |
| Sterility (Ph. Eur. 2.6.1, USP <71>) Pyrogens/endotoxin Rabbit Pyrogen Test (Ph. Eur. 2.6.8, USP<151>) Limulus amoebocyte lysate (LAL) bacterial endotoxin test (Ph. Eur. 2.6.14, USP<85>) | Sterility test for finished products endotoxin test for raw materials, pharmaceuticals waters, finished products |
| Antimicrobial preservative efficacy testing (Ph. Eur. 5.1.3, USP <51>) | Antimicrobial preservative efficacy |
| Microbiological assay of antibiotics (E.P 2.7.2., USP<81>) | Assays of antibiotic |
| USP <1112> Application of water activity determination to nonsterile pharmaceutical products | Water activity can be used to predict microbial proliferation in the product |
| USP <1211> Sterilization and sterility assurance of compendial articles. There are a series of subchapters that describe specific sterilization methods | Sterilization and microbial reduction |
| USP <55> Biological indicators | Biological indicators for assessing microbial reduction |
| USP <1113> Microbial characterization, identification, and strain typing | Microbial identification |
| USP <1117> Microbiological best laboratory practice | Management of microbiological good laboratory practice |
| USP <1116> Microbiological control and monitoring of aseptic processing environments | Environmental monitoring for aseptic environments |
| Ph. Eur. 5.1.6 Alternative methods for control of microbiological quality/ USP <1223> Validation of alternative microbiological methods | Alternative methods/ Rapid microbiological methods |
| USP <1115> Bioburden control of nonsterile drug products | Bioburden control |
| USP <1227> Validation of microbial recovery from pharmacopeial articles | Microbial method validation |
3. Which factors of culture media affects the cultivation of microorganisms?
Culture media factors affecting the cultivation of microorganisms are optimum nutrients, oxygen or other gases, moisture, pH, and temperature.
4. What are the important nutrients of culture media that affects the cultivation of microorganisms?
The key nutrients of culture media that affects the cultivation of microorganisms are sources of carbon, nitrogen, water, inorganic phosphates and sulfur, vitamins, and trace metals.
5. Whose name recognize as “The Father of Culture Media”?
Robert Koch (1843–1910) discovered that broths based on fresh beef serum or meat extracts (so-called bouillons, the term “broth” for liquid culture medium being analogous to broth or soup) produced optimal growth.
6. Who was the first scientist cultivated the microorganisms on a growth medium?
The French chemist and microbiologist, Louis Pasteur (1922–1985).
7. Petri dish is named based on which scientists’ contribution?
Julius Richard Petri (1852–1921)
8. What are the different types of culture medium used in pharmaceuticals and what is the purpose of those?
| Types of medium | Use |
| Nutrient agar or broth and tryptone soya agar or broth. Tryptone soya agar (equivalent to soyabean casein digest medium) | → Environmental monitoring→ Isolation and cultivation of nonfastidious and fastidious microorganismsNote: Fastidious bacteria are bacteria that need special nutritional supplements and conditions to grow on agar plates. Nonfastidious bacteria are bacteria that do not need special nutritional supplements and conditions to grow on agar plates |
| Tryptone soya broth | Sterility testing and as a general growth broth in microbial enumeration tests, as well as used for media simulation trials |
| vegetable peptone broth | Media filling trials |
| fluid thioglycollate medium | used for the growth of bacteria (aerobic and anaerobic) as a part of the sterility test |
| fungi | Sabouraud dextrose agar or malt extra agar |
| R2A | Microbiological examination of water (This is a low nutrient agar used for the cultivation of heterotrophic microorganisms). |
| Columbia blood agar | Detection of hemolytic reactions by Staphylococci |
9. What is the process of Growth Promotion test of microbiology culture media? What should be the acceptance criteria?
1. Medium to be inoculated with a microorganisms <100 Colony Forming Units (CFU).
2. Use not less than five unique strains as recommended by the pharmacopoeias and two organisms from environment isolates on rotation basis (It should be within the five passages from the original reference culture seed lot).
3. Compare the growth on the medium with medium previously approved culture lot.
4. Acceptance is no more than a factor of 2 differences in productivity ratio calculations.
10. What is the calculation of productivity ratio and what is the acceptance criterion for agar media plate or solid media for growth promotion test?
Productivity ratio =
Mean of two test plates (cfu)/ Mean of two comparative control plates (cfu)
An acceptable productivity ratio should be (0.5 – 2.0). That is equivalent to a 50 – 200 % recovery.
11. What are the quantitative techniques of Growth Promotion Tests for Solid media?
Quantitative techniques of Growth Promotion Tests are of two types.
● Ecometric
● Miles – Misra (Drop Count)
12. Explain ecometric method of Growth Promotion Tests?
This technique is semi-quantitative variant of the streaking.
This technique is operator-dependent and has lower precision. However, it can be used to great effect with practice.
Following are the steps:
● A fresh suspension of the challenge organism is taken into a calibrated loop (One loopful of inoculm).
● Five streaks are streaked out into four quadrants onto the agar plate along with a final streak in the center of the plate.
● These plates are then incubated overnight for growth.
● The patterns of growth are interpreted to provide an Absolute Growth Index (AGI)
13. Explain Miles – Misra technique (the drop count technique) of Growth Promotion Tests?
This technique involves spreading droplets of known quantities (10 μL) of microbial suspensions.
The test plate is compared against control plate after incubation to verify number of colonies recovered.
Note:
The accuracy of the method is dependent on following factors:
● Dilution used
● Number of colony forming units (cfu) in the inoculum
● Volume of the inoculums
● Spreading technique
14. What are the quantitative techniques of Growth Promotion Tests for Broth Media?
Quantitative techniques of Growth Promotion Tests for Broth Media are of four types.
● Copious Growth
● End-point Methods
● Most Probable Number (MPN)
● Kinetic Parameters
15. Explain Copious Growth method of Growth Promotion Tests for Broth Media?
This is the method of choice as per compendial. The challenge of broth media is done by comparing the growth over the period of time with a control batch (which provides a qualitative assessment of copious growth). In this method, the challenge organism is inoculated at a very low level (< 100 CFU per unit) and incubated at the prescribed temperature for the prescribed period of time (3 days or 5 days).
The advantage of this method is that it does not require a great deal of labor. Semi-quantitative assessment can be done by constructing a growth index from slight to copious growth (normally a scale of +, ++, or +++).
16. Explain End-point Methods method of Growth Promotion Tests for Broth Media?
In this method, very low levels of inoculum are added to multiple tubes of the two media under testing. Growth frequency is compared between the two media to understand the equivalency.
17. Explain Most Probable Number (MPN) method of Growth Promotion Tests for Broth Media?
This is a Microbial Limits Test. In this method, the unknown sample is prepared in a ten-fold dilution series and added to nutrient broth in replicate tubes. The tubes will then either turn turbid (growth) or remain clear, and allow for an estimate of the most probable number of microorganisms.
18. Explain Kinetic Parameters method of Growth Promotion Tests for Broth Media?
The growth promoting testing of two lots of broth can be compared by measuring the growth curves of same inocula grown side-by-side. The growth rate of an organism can be determined by spectrophotometrically or by viable count.
19. How Growth Promotion Tests for selective media is performed?
Selective media requires a different approach than general purpose media. The objective of the test for selective media is to see that the media supports the growth of specific microorganisms. Inhibition, colony morphology and pigment are verified during the testing. In order to determine growth of selective media, specific microorganisms are used as positive or negative indicators.
20. What are the steps involved in the manufacturing of culture media?
a. Collection of prerequisite
● Decide batch size
● Vessel for holding or dispensing the media
● Weighing balance
● Petri dishes/ Containers
● Dehydrated media
b. Rehydration of media
● Media powder is rehydrated by mixing the medium in the volume of water (Amount should be based on manufacturer’s instruction)
● Homogenize the solution by mixing
● Care must be taken to avoid scorching the media.
● The media should clarify near boiling (95–100 °C), and the media should only be allowed to boil less than 1 min.
c. Sterilization
d. Addition of supplements (depending on the types of media requirement)
e. Filling in container
f. Status labeling
21. What are the acceptance criteria for microbiological quality of non-sterile dosage forms and raw materials?
| Application | TAMC | TYMC | Specified microorganisms |
| Non-aqueous preparations for oral use | 1000 | 100 | Escherichia coli absent in 1 g or 1 mL |
| Aqueous preparations for oral use | 100 | 10 | Escherichia coli absent in 1 g or 1 mL |
| Rectal use | 1000 | 100 | Need based |
| Oromucosal use Gingival use Cutaneous use Nasal use Auricular use | 100 | 10 | Staphylococcus aureus absent in 1 g or 1 mL Pseudomonas aeruginosa absent in 1 g or 1 mL |
| Vaginal use | 100 | 10 | Staphylococcus aureus absent in 1 g or 1 mL Pseudomonas aeruginosa absent in 1 g or 1 mL Candida albicans absent in 1 g or 1 mL |
| Inhalation use | 100 | 10 | Staphylococcus aureus absent in 1 g or 1 mL Pseudomonas aeruginosa absent in 1 g or 1 mL Bile-tolerant gram-negative bacteria absent in 1 g or 1 mL |
| Transdermal patches | 100 | 10 | Staphylococcus aureus absent in 1 g or 1 mL Pseudomonas aeruginosa absent in 1 g or 1 mL |
| Raw material for pharmaceutical use | 1000 | 100 | Need based |
22. What are the risks because of microorganism in the pharmaceutical formulation if not within the acceptable limit?
● Decomposition of the product
● Degradation of product
● Infection to the patient
23. Give few examples of good practice in a microbiological laboratory.
● Required equipment and media should sterilized prior to use.
● Sterilized equipment and media should be maintained and stored in such a way that it should not be contaminated.
● Frequently disinfect hands and working surfaces while carryout aseptic operations.
● Fly, rodent and pest control.
● Use personnel protective equipment such as laboratory coat, safety glasses, and gloves.
● Drinkable and eatables should not be allowed in the laboratory.
● Sterilize contaminated used media and waste prior to disposal.
● Pipetting should not be done mouth.
24. Why aseptic technique should be followed in the microbiological laboratory?
To prevent contamination and cross-contamination in microbiology laboratory. Probability of contamination in the laboratory is – samples, media, environment, facility etc.
25. How asepsis can be achieved in the laboratory?
● Frequent washing and disinfecting hands.
● Use of unidirectional airflow station for critical operations.
● Handling of positive control at the end of analysis.
● Not touching the samples and accessories directly by hand.
26. What is pure culture?
A pure culture is a population of cells or multicellular organisms growing in the absence of other species or types in which cells are genetic clones of one another.
27. What is most common method to isolate individual cells and produce a pure culture?
The most common method to isolate individual cells and produce a pure culture is to prepare a streak plate method.
28. Explain Streak Plate Method.
a. Sterilize the inoculating loop by heating it until red hot in a flame until it is red hot. Allow it to cool.
b. Pick up a loop full of liquid inoculum or bacterial growth from the surface of an agar plate and, starting about 2.5 cm in from the edge of the plate, streak lightly back and forth with the loop flat, making close, parallel streaks back to the edge of the plate to a quarter of the plate.

c. Sterilize the loop and cool again, going back to the edge of area first quarter that you just streaked, extend the streaks into the second quarter of the plate.
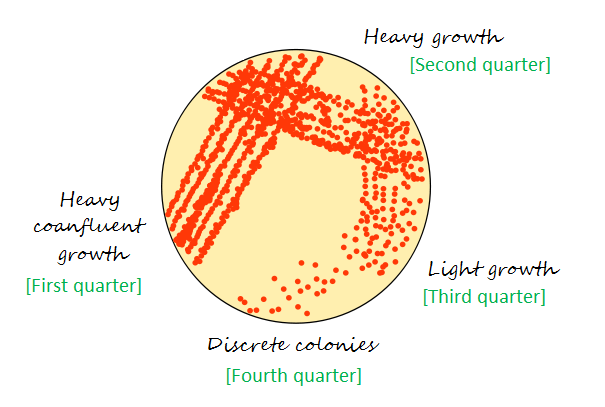
d. Repeat the same process for remaining two quarter of the plate.
e. Flame and cool the loop again.
29. What should be done during microbiology testing if sample exhibit antimicrobial activity?
When the sample possesses antimicrobial activity, it that requires neutralization. Following are commonly used principle for neutralization:
● Chemical neutralization
● Enzymatic neutralization
● Dilution
30. What is Pyrogens and endotoxins?
Pyrogens and endotoxins are a heterogeneous group of chemical entities that cause fever when injected.
31. What is the source of pyrogens and endotoxins?
Pyrogens can be nonbacterial as well as bacterial in origin.
In the pharmaceutical industry, mostly observed source is from Gram-negative bacteria. That is the lipopolysaccharide (LPS) from the bacterial cell wall.
Pyrogens are the metabolic byproduct of the Gram-negative bacteria.
32. Explain types of microbial identification methods.
Identification methods can be divided into two types:
● Phenotypic
● Genotypic
33. What are the differences between Phenotype and Genotype?
● “Genotype” is an organism’s full hereditary information.
● “Phenotype” is an organism’s actual observed properties, such as morphology, development or behavior.
34. What are the different staining techniques used in the pharmaceutical microbiology laboratory?
● Gram-stain
● Bacterial spore stain
● Fungal staining
● Ziehl–Neelsen stain
35. What is the objective of microbial identification?
The objective of microbial identification is to differentiate one microbial isolate from another with respect to family (genus) and a species or a particular strain.
36. What are the taxonomic terms for microbial identification?
● Family: group of similar genera
● Genus: group of similar species
● Species: group of similar strains
● Type: strains within a species
● Strain: an isolate of a particular species
37. What is the first step of microbial identification?
The first step of most identification program is colony and cellular morphology of the microorganism.
Colony morphology is a method that used to describe the characteristics of an individual colony of bacteria on agar media in a Petri dish.
Colony morphology is normally classified based on the form, elevation and margin. This can be further classified as follows.
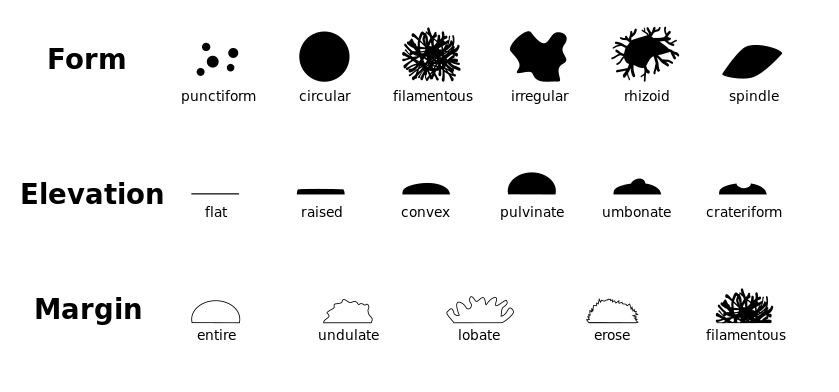
38. What is Gram-stain?
The Gram stain is an important technique for identification of bacteria. It divides bacteria into two groups, Gram-positives and Gram-negatives.
39. How the Gram-staining technique is useful?
Gram-staining technique that allows to visualize the morphological types of bacteria using a compound light microscope under magnification of 100x.
40. Explain the principle of the Gram-staining technique?
Step 1: Crystal violet (primary stain) aqueous solutions consist of +ve and –ve ions. These ions penetrate through the cell wall and cell membrane of all types of bacteria. The +ve ion stains the bacterial cells and stains the cells purple.
Step 2: Iodide (the mordant) interacts with crystal violate and forms complexes.
Step 3: Decolorizer (made of acetone and alcohol 95%) interacts with the lipids of the cell membrane. A Gram-negative cell loses its outer lipopolysaccharide membrane, and the inner peptidoglycan layer is left exposed.
On the other hand, a Gram-positive cell becomes dehydrated because of property of decolorizer. The complexes trapped within the Gram-positive cell due to the multilayered nature of the peptidoglycan.
Step 4: When decolorization is added, the Gram-positive cell remains purple, and the Gram-negative cell loses its purple color.
Step 5: A Safranin, the counterstain is used to provide color to Gram-negative bacteria a pink/ red color.

41. What is the procedure of Gram-staining technique?
a. Take a clean, grease free slide.
b. Prepare the smear of suspension on the clean slide with a loopful of sample.
c. Air-dry or heat-fix smear of cells for around 1 minute with crystal violet staining reagent. (Note: Too heavy or too light cell concentration will affect the Gram Stain results.)
d. Rinse the slide with gentle stream of tap water for 2-3 seconds.
e. Flood the gram’s iodine for 1 minute and wash with gentle stream using tap water for 2-3 seconds.
f. Flood slide with 95% alcohol or acetone for about 15-20 seconds until decolorizing agent running from the slide shows clear liquid.
g. Add counterstain, safranin and wait for 1 minute
h. Wash slide in a gentile stream of tap water until it appear colorless.
i. Blot dry with absorbent paper.
j. Observe under oil immersion using a Brightfield microscope.
k. Results:
Gram-negative bacteria will stain pink or red
Gram-positive bacteria will stain blue or purple
42. What is the procedure of Gram-staining technique?
a. Primary Stain: Crystal Violet Staining Reagent.
Solution A for crystal violet staining reagent
Crystal violet (90% dye content) – 1g
2g Ethanol, 95% (v/v) – 10 ml
Solution B for crystal violet staining reagent
Ammonium oxalate, 0.4 g
Distilled water, 40 ml
Mix solution A and solution B to obtain crystal violet staining reagent. Store for 1 day and then filter using filter paper.
b. Mordant: Gram’s Iodine
Iodine, 0.5 g
Potassium iodide, 1.0 g
Distilled water, 150 ml
Triturate iodine and potassium iodide in a mortar and add water slowly with continuous trituration until the iodine is dissolved. Store in amber bottles.
c. Decolorizing Agent
Ethanol, 95% (vol/vol)
Alternate decolorizing agent – 1:1 acetone and ethanol mixture.
d. Counterstain: Safranin
Use 1.25g Safranin O and mix it with 50 ml 95% Ethanol (Solution A). Take this Solution A 5 ml and mix it with 45 ml distilled water
43. Which indicators are used for spore staining?
Malachite green (a triarylmethane dye) and a safranin (an azonium compound) counterstain is useful tool in identifying the presence or absence of spores.
This is referred to as the Schaeffer-Fulton stain.
44. Explain the Schaeffer-Fulton staining method for endospore staining technique?
1. Prepare a bacterial smear on a clean slide, air dry and gently heat fix.
2. Cover the slide with a piece of paper towel, and place on a staining rack over the water bath with boiling water.
3. Flood the paper towel on the slide with Malachite green (primary stain). Steam the slide for about 5 minutes.
4. Remove the slide from the water bath, and remove the paper towel from the slide.
5. Allow the slide to cool, and then rinse with deionized water until the water runs clear.
6. Drain excess water and apply Safranin (counterstain) for 2 minutes.
7. Rinse Safranin off with deionized water, and blot the slide dry with blotting paper.
8. Examine the slide with a light microscope under oil immersion objective
Result:
Endospores appears green
Vegetative cells appear red or pink

45. What is Lactophenol cotton blue stain or fungal staining?
Lactophenol cotton blue stain is used to examining yeast and filamentous fungi under microscope.
Phenol acts as a disinfectant to kill living organisms
Lactic acid used to preserve the fungal structures
Cotton blue is used to stain fungal cell wall and other fungal structures
On staining, it fungus will be with blue colored spores and structures, such as hyphae.
The identification of fungi using macroscopic and microscopic techniques is difficult and requires a trained eye.


46. What reagents used to prepare Lactophenol cotton blue staining solution?
Distilled water
Cotton Blue or Aniline Blue
Phenol Crystals
Glycerol
Lactic acid
70% ethanol
47. What is use of Ziehl-Neelsen stain?
The objective of Ziehl-Neelsen stain is to differentiate bacteria into acid fast group and non-acid fast groups.
48. What Acid-Fast stain called as Ziehl-Neelsen stain?
This technique was first developed by Ziehl and later on modified by Neelsen, therefore, this method is also called as Ziehl-Neelsen staining techniques.
| Reagents | Color of Acid fast | Color of Non-acid fast |
| Primary dye – Carbol fuchsin | Red | Red |
| Decolorizer – Acid alcohol | Red | Colorless |
| Counter stain – Methylene blue | Red | Blue |

49. What is Total viable aerobic count?
Total viable aerobic count is designed to count the number of microorganisms (as colony forming units, CFUs) in a non-sterile pharmaceutical product or raw materials.
It is father divided into two parts, total aerobic microbial count (TAMC) and total yeast and mould counts (TYMCs).
50. Which are the methods used for analysis of total viable aerobic count?
● Membrane filtration technique – Sample is filtered and the filter is placed on defined media
● Pour plate technique – Sample aliquot is taken and placed in a Petri dish and specified media poured onto the sample
● Spread plate technique – Sample aliquot is placed on the surface of defined media and smeared evenly over the surface
● Most probable number (MPN) technique – This method is used for mainly insoluble materials. The sample dilutions are placed into a series of replicate tubes and the number of tubes showing growth give a statistical evaluation of the number of microorganisms in the sample.
51. Which is Bioburden test Method Validation?
The Bioburden Method Validation is done to demonstrate the adequacy of sample preparation method and the ability of the media to recover microorganisms in the presence of the test sample. Following should be considered during the method validation.
(a) Growth promotion of media
(b) Sample preparation
(c) Test method (d) Sample neutralization
52. Which are the various organization from where approved culture collections of different train of microorganisms can be obtained?
● American Type Culture Collection (ATCC)
● National Collection of Industrial and Marine Bacteria (NCIMB)
● Collection of Institute Pasteur (CIP)
● Imperial Mycological Institute (IMI)
● National Collection of Pathogenic Fungi (NCPF)
● National Biologicals Resources Centre (NBRC)
53. Explain the sample preparation process for Bioburden test.
● Sample handing area: Sample should be prepared on a laboratory bench, within unidirectional airflow cabinet or an isolator.
● Bacteria cultures handling area: Biosafety cabinet or Microbiological Safety Cabinet should be used.
● Sample preparation for Water-soluble products:
a. Dissolve or dilute the product with ration of 1 in 10 dilution in phosphate buffer solution pH 7.2, If necessary, adjust to a pH of 6 – 8.
b. If required, further dilutions can be done to get not more than 250 CFU/plate in case of TAMC, 50 CFU/plate in case of TYMC.
c. If needed, to dissolve the sample completely, triturate it in a sterile mortar and pestle in an aseptic environment to get a fine powder.
● Sample preparation for Non-fatty and insoluble material in water:
a. Suspend the product with 1 in 10 dilution in phosphate buffer solution pH 7.2.
b. A surfactant such as 1 g/L of polysorbate 80 can be used to assist the suspension of poorly wettable substances.
c. If require, adjust to a pH of 6–8.
d. If required, further dilutions can be done to get not more than 250 CFU/plate in case of TAMC, 50 CFU/plate in case of TYMC.
● Sample preparation for fatty products:
a Dissolve in isopropyl myristate sterilized by filtration, or mix the product to be examined with the minimum necessary quantity of sterile polysorbate 80.
b. Heat if required for NMT 40 °C or, in exceptional cases, to not more than 45 °C and maintain the temperature in a water bath.
c. Add pre-warmed diluent to make a 1 in 10 dilution of the product.
d. Form of an emulsion.
c. If needed, further serial tenfold dilution can be prepared using the diluent containing a suitable concentration of sterile polysorbate 80 or another non-inhibitory sterile surfactant.
54. Explain the method of measurement of microbial concentration in suspension by optical density?
A spectrophotometer method is used to measures turbidity of inoculum suspension. When light passes through a suspension of microorganisms, light gets scattered and the amount of scatter is an indication of the concentration present in the suspension.
While doing the estimation using this method, a calibration curve needs to be constructed using series of the known concentration. Based on the calibration curve, unknown concentration can be easily identified.
55. What are the common sources of pyrogens?
Non-bacterial source:
● Antigens
● Poly nucleotides
● Steroids
● Adjuvants
● Viruses
● Fungi
Bacterial source:
● Streptococcal toxins
● Staphylococcal enterotoxins
● Mycobacterial cell wall components
● Bacterial cell wall – lipopolysaccharides (endotoxins)
56. What are the method used to test pyrogens and endotoxins?
● Rabbit pyrogen test
● Limulus Amoebocyte Lysate (LAL) testing for bacterial endotoxin
57. Is sterility test qualitative or quantitative test?
The sterility test is a qualitative test. Results are written as presence or absence of turbidity based on growth for bacteria and fungi in media.
58. Which are commonly used sterility testing methods?
● Membrane filtration: This is further classified as open method and closed system method.
● Direct inoculation method
59. What components required performing sterility testing by membrane filtration method?
● Filter with pore size 0.45 μm
● Filter diameter about 50 mm
● Cellulose nitrate filters for aqueous, oily, and weak alcoholic solutions
● Cellulose acetate filters for strong alcoholic solutions
60. What are the growth mediums used to perform sterility testing?
● Fluid Thioglycollate Medium for anaerobic bacteria and it also isolates aerobic bacteria
● Soya-bean Casein Digest Medium for the isolation of fungi and aerobic bacteria
61. What types of growth promotion test (GPT) to be done for growth medium used to perform sterility testing?
● GPT for Fluid thioglycollate medium shall be done using Clostridium sporogenes, P. aeruginosa, S. aureus;
● GPT for Soya-bean casein digest medium shall be done using A. niger, B. subtilis, C. albicans.
The medium shall be inoculated with not more than 100 CFU and incubated for 3 days (bacteria) or 5 days (fungi).
Result: Clearly visible growth must be observed.
62 What should be incubation conditions and times for sterility testing?
Incubation conditions and times for sterility testing are as follows:
● Fluid Thioglycollate Medium at 30 – 35 °C
● Soya-bean Casein Digest Medium at 20 – 25 °C ● Total incubation period of 14 days with visual examination for turbidity.
63. How to interpret sterility test validation outcome?
There are two outcomes with the sterility test:
(1) Clearly visible growth – test sample and the control tubes are equivalent
(2) No clearly visible comparable growth – Test needs to be modified and the validation needs to be repeated
64. What should be the environment for sterility testing?
The sterility test environment should be EU GMP Grade A with a Grade B background or a Grade A isolator operator with background Grade C or D.
65. How to collect sterility testing sample during the batch?
The test samples for sterility testing needs to be representative of the batch and entire filling operation that is, beginning, middle, and end of the aseptic fill process. Additionally, sterility test sample required to be collected when unplanned intervention occurred during the batch.
66. What is the composition of Fluid thioglycollate medium?
The composition of Fluid thioglycollate medium is as follows:
| Name of ingredient | Weight |
| L-Cystine | 0.5 g |
| Agar | 0.75 g |
| Sodium chloride | 2.5 g |
| Glucose monohydrate/anhydrous | 5.5/5.0 g |
| Yeast extract (water-soluble) | 5.0 g |
| Pancreatic digest of casein | 15.0 g |
| Sodium thioglycollate or | 0.5 g |
| Thioglycollic acid | 0.3 ml |
| Resazurin sodium solution (1 g/l of resazurin sodium), freshly prepared | 1.0 ml |
| Water R | 1000 ml |
pH after sterilization 6.9 to 7.3.
67. What is the composition of Soya-bean casein digest medium?
The composition of Soya-bean casein digest medium is as follows:
| Name of ingredient | Weight |
| Pancreatic digest of casein | 17.0 g |
| Papaic digest of soya-bean meal | 3.0 g |
| Sodium chloride | 5.0 g |
| Dipotassium hydrogen phosphate | 2.5 g |
| Glucose monohydrate/anhydrous | 2.5/2.3 g |
| Water R | 1 000 ml |
pH after sterilization 7.1 to 7.5.
68. While sterility testing using membrane filtration, what should be suitable volume of diluent and sample quantity of product to be examined prescribed?
Liquids sample
| Minimum quantity to be used for each medium Quantity per container | Minimum quantity to be used for each medium unless otherwise justified and authorized |
| • less than 1 ml | The whole contents of each container |
| • 1-40 ml | Half the contents of each container but not less than 1 ml |
| • greater than 40 ml and not greater than 100 ml | 20 ml |
| • greater than 100 ml | 10 per cent of the contents of the container but not less than 20 ml |
| Antibiotic liquids | 1 ml |
Insoluble preparations, creams and ointments sample
| Minimum quantity to be used for each medium Quantity per container | Minimum quantity to be used for each medium unless otherwise justified and authorized |
| Insoluble preparations, creams and ointments to be suspended or emulsified | Use the contents of each container to provide not less than 200 mg |
Solids sample
| Minimum quantity to be used for each medium Quantity per container | Minimum quantity to be used for each medium unless otherwise justified and authorized |
| • less than 50 mg | The whole contents of each container |
| • 50 mg or more but less than 300 mg | Half the contents of each container but not less than 50 mg |
| • 300 mg – 5 g | 150 mg |
| • greater than 5 g | 500 mg |
Reference: WHO, Document QAS/11.413 FINAL, March 2012
69. Types of filter preferable for sterility testing using filtration method.
Cellulose nitrate filters: For aqueous, oily and weakly alcoholic solutions
Cellulose acetate filters: Strongly alcoholic solutions
70. What is preferred filter diameter for sterility testing using filtration method?
50 mm in diameter
71. Explain Membrane filtration method for sterility testing?
● The filtration apparatus and membrane are sterilized by appropriate means.
● Use membrane filters with nominal pore size not greater than 0.45 μm and diameter 50 mm.
● Solution to be examined shall be filtered under aseptic conditions.
● Aseptically remove the membrane and transfer to the medium.
Method for aqueous solutions:
● Transfer a small quantity of a suitable, sterile diluent such as a 1 g/l neutral solution of meat or casein peptone pH 6.9 to 7.3 onto the membrane in the apparatus and filter.
● Use diluent containing suitable neutralizing substances and/or inactivating substances in the case of antibiotics.
● Transfer the contents of the container or containers to be tested to the membrane or membranes, if necessary after diluting to the volume used in the method suitability test with the chosen sterile diluent but in any case using not less than the quantities of the product to be examined prescribed in Table-1.
● Filter immediately.
● If the product has antimicrobial properties, wash the membrane not less than three times by filtering through it each time the volume of the chosen sterile diluent used in the method suitability test.
● Do not exceed a washing cycle of five times 100 ml per filter, even if during method suitability it has been demonstrated that such a cycle does not fully eliminate the antimicrobial activity.
● Transfer the whole membrane to the culture medium or cut it aseptically into two equal parts and transfer one half to each of two suitable media.
● Use the same volume of each medium as in the method suitability test.
● Alternatively, transfer the medium onto the membrane in the apparatus. Incubate the media for not less than 14 days.
Soluble solids:
Use for each medium not less than the quantity prescribed in Table 3 of the product dissolved in a suitable solvent such as the solvent provided with the preparation, water for injections R, sodium chloride (9 g/l) TS or peptone (1 g/l) TS1 and proceed with the test as described above for aqueous solutions using a membrane appropriate to the chosen solvent.
Oils and oily solutions:
● Use for each medium not less than the quantity of the product prescribed in Table 2.
● Oils and oily solutions of sufficiently low viscosity may be filtered without dilution through a dry membrane.
● Viscous oils may be diluted as necessary with a suitable sterile diluent such as isopropyl myristate R shown not to have antimicrobial activity in the conditions of the test.
● Allow the oil to penetrate the membrane by its own weight then filter, applying the pressure or suction gradually.
● Wash the membrane at least three times by filtering through it each time about 100 ml of a suitable sterile solution such as peptone (1 g/l) TS1 containing a suitable emulsifying agent at a concentration shown to be appropriate in the method suitability test, for example polysorbate 80 at a concentration of 10 g/l.
● Transfer the membrane or membranes to the culture medium or media or vice versa as described above for aqueous solutions, and incubate at the same temperatures and for the same times.
Ointments and creams:
● Use for each medium not less than the quantities of the product prescribed in Table 2.
● Ointments in a fatty base and emulsions of the water-in-oil type may be diluted to 1 per cent in isopropyl myristate R as described above, by heating, if necessary, to not more than 40 °C.
● In exceptional cases it may be necessary to heat to not more than 44 °C.
● Filter as rapidly as possible and proceed as described above for oils and oily solutions.
Table: 1 – Liquids sample
| Minimum quantity to be used for each medium Quantity per container | Minimum quantity to be used for each medium unless otherwise justified and authorized |
| • less than 1 ml | The whole contents of each container |
| • 1-40 ml | Half the contents of each container but not less than 1 ml |
| • greater than 40 ml and not greater than 100 ml | 20 ml |
| • greater than 100 ml | 10 per cent of the contents of the container but not less than 20 ml |
| Antibiotic liquids | 1 ml |
Table: 2 – Insoluble preparations, creams and ointments sample
| Minimum quantity to be used for each medium Quantity per container | Minimum quantity to be used for each medium unless otherwise justified and authorized |
| Insoluble preparations, creams and ointments to be suspended or emulsified | Use the contents of each container to provide not less than 200 mg |
Table: 3 – Solids sample
| Minimum quantity to be used for each medium Quantity per container | Minimum quantity to be used for each medium unless otherwise justified and authorized |
| • less than 50 mg | The whole contents of each container |
| • 50 mg or more but less than 300 mg | Half the contents of each container but not less than 50 mg |
| • 300 mg – 5 g | 150 mg |
| • greater than 5 g | 500 mg |
Reference: WHO, Document QAS/11.413 FINAL, March 2012
72. Explain the direct inoculation of the culture medium for sterility testing?
● Transfer the quantity of the preparation to be examined prescribed in the following Table directly into the culture medium so that the volume of the product is not more than 10% of the volume of the medium, unless otherwise prescribed.
Table: 1 – Liquids samples
| Minimum quantity to be used for each medium Quantity per container | Minimum quantity to be used for each medium unless otherwise justified and authorized |
| • less than 1 ml | The whole contents of each container |
| • 1-40 ml | Half the contents of each container but not less than 1 ml |
| • greater than 40 ml and not greater than 100 ml | 20 ml |
| • greater than 100 ml | 10 per cent of the contents of the container but not less than 20 ml |
| Antibiotic liquids | 1 ml |
Table: 2 – Insoluble preparations, creams and ointments samples
| Minimum quantity to be used for each medium Quantity per container | Minimum quantity to be used for each medium unless otherwise justified and authorized |
| Insoluble preparations, creams and ointments to be suspended or emulsified | Use the contents of each container to provide not less than 200 mg |
Table: 3 – Solids samples
| Minimum quantity to be used for each medium Quantity per container | Minimum quantity to be used for each medium unless otherwise justified and authorized |
| • less than 50 mg | The whole contents of each container |
| • 50 mg or more but less than 300 mg | Half the contents of each container but not less than 50 mg |
| • 300 mg – 5 g | 150 mg |
| • greater than 5 g | 500 mg |
● If the product to be examined has antimicrobial activity, carry out the test after neutralizing this with a suitable neutralizing substance or by dilution in a sufficient quantity of culture medium.
● When it is necessary to use a large volume of the product it may be preferable to use a concentrated culture medium prepared in such a way that it takes account of the subsequent dilution.
● Where appropriate the concentrated medium may be added directly to the product in its container.
Oily liquids:
● Use media to which have been added a suitable emulsifying agent at a concentration shown to be appropriate in the method suitability of the test, for example polysorbate 80 at a concentration of 10 g/l.
Ointments and creams:
● Prepare by diluting to about 1 in 10 by emulsifying with the chosen emulsifying agent in a suitable sterile diluent such as peptone (1 g/l) TS1.
● Transfer the diluted product to a medium not containing an emulsifying agent.
● Incubate the inoculated media for not less than 14 days. Observe the cultures several times during the incubation period.
● Shake cultures containing oily products gently each day. However when fluid thioglycollate medium is used for the detection of anaerobic microorganisms keep shaking or mixing to a minimum in order to maintain anaerobic conditions.Reference: WHO, Document QAS/11.413 FINAL, March 2012


