Complete guide: High Performance Liquid Chromatography (HPLC) – History, Definition, Principle, Types, Instrumentation, and Applications
History of Column Chromatography and High Performance Liquid Chromatography (HPLC)
History of Column Chromatography or Liquid Chromatography
To understand the history of HPLC, we first needs to understand the history of Liquid chromatography. Liquid chromatography was invented in the early 1900s by the Russian botanist, Mikhail S. Tswett, born in 1872 in Italy, during his research on plant pigments. His studies mainly focused on separating leaf pigments using a solvent in a column packed with particles.
The scientist used a glass column filled with calcium carbonate and aluminum oxide and passed the solvent extract of plant leaves through the column. Subsequently, the pure solvent was passed through the column. As a result, colored bands are observed separating. It has happened because some components of plant extracts were moving faster than others.
Using this technique, he had separated different compounds. The compounds that have strong attracted to the particles filled in the columns passed downwards slowly compared to those which were more strongly attracted to the solvent and moved faster.
In this technique, the moving solvent is called the mobile phase, and the particles are called the stationary phase.
Mikhail Tswett named this technique as chromatography. Chroma means color in the Greek language, and Graph means writing.
The modern definition of chromatography is, it is a physicochemical technique of separation in which the compounds that required to be separated are distributed between two phases, one is called stationary phase (which remains stationary), and the other is a mobile phase (which moves through the stationary phase). The separation happens on the basis of their molecular structure and molecular composition.
Reverse Phase HPLC system is most commonly and widely method used in the pharmaceuticals and bio-pharmaceuticals
History of High Performance Liquid Chromatography
High Performance Liquid Chromatography (HPLC) is also known as high-performance liquid chromatography or high pressure liquid chromatography. This technique is a modified column chromatography technique.
The Column Chromatography or Liquid Chromatographic systems were a time-consuming method of separation due to the lower solvent flow rate because it was mainly dependent on gravitational force.
Between 1941 and 1960, scientists predicted that the liquid chromatography could be operated with high efficiency by reducing the column packing particle size to around 150 μm and flowing the mobile phase at increased velocity.
Between 1960 to 1970, extensive scientific work has been carried out by scientists to improve liquid chromatography. As a result, in the 1970s, many developments were seen around instrumentation and hardware.
Scientists started using high pressure pumps and injectors to make a basic design of an HPLC system.
Principle of Separation in High Performance Liquid Chromatography (HPLC)
Column chromatography has three main components. Stationary phase, Mobile Phase, and a sample from which components need to be separated.
What is a Stationary Phase: Unlike its name, it is the phase that does not move during the experimentation or analysis. Instead, it retains and reduces the flow of the components within the sample to be tested based on its affinity to the stationary phase, and the compound gets separated at different times.
What is Mobile Phase: It is a solvent or mixture of solvent that does move through the stationary phase. As it continuously flows through the stationary phase, it takes the compounds with it to separate the components of the sample.
A component that has a high affinity towards the mobile phase will elute quicker from the stationary phase. However, a component that has a high affinity with the stationary phase (column) will elute slower. The affinity of components means chemical attraction.
As a general rule, modes of separation in HPLC mainly depend on three factors; those are:
(i) Polarity
(ii) Molecular Size
(iii) Electrical Charge
Types of High Performance Liquid Chromatography (HPLC)
There are the following types of HPLCs, depending upon the phase system (stationary) in the process :
1. Normal Phase HPLC (NP HPLC)
This method utilizes a polar stationary phase and non-polar mobile phase to separate analytes on the basis of polarity. An example of polar bonding is hydrogen-bonding or dipole-dipole type of interaction. In this setup, the generally used stationary phase is silica, and example mobile phases are methylene chloride, diethyl ether, hexane, chloroform, or mixture in different ratios.
In this technique, polar components in the analyte elute slower than the non-polar components. Therefore, adsorbent strength can be increased by increasing the component polarity, and elution time increase the interaction between component and stationary phase.
2. Reverse Phase HPLC (RP-HPLC or RPC)
Reverse Phase HPLC method utilizes a nonpolar (hydrophobic) stationary phase and polar mobile phase to separate analytes on the basis of polarity. In this setup, the generally used stationary phase is bonded silica, and example mobile phases are Water, Methanol, Buffers, Acetonitrile, etc.
Reverse Phase HPLC technique works on the principle of hydrophobic interactions between component and stationary phase; hence, the nonpolar material is retained longer than the polar component. RP-HPLC is the most common technique to analyze pharmaceutical products in the pharmaceutical industry.
3. Size-exclusion HPLC (SEC) or Gel Permeation or Filtration Chromatography
Size-exclusion chromatography is basically a simple molecule size classification process. More significant molecular weight components elute first, and smaller molecular size materials elute then after. A column is filled with a porous material. It has controlled pore size, and particles are separated as per molecular size.
The sample molecules that are too large to diffuse into the pores between the individual stationary phase particles get excluded. The small molecules to penetrate the pores are present, and then the entire mobile phase volume becomes available to them.
4. Ion-Exchange HPLC
Ion-exchange chromatography separation technique works based on the electrical charge on the stationary phase and components in the sample. The stationary phase surface is ionically charged with opposite ions to the sample ions. This method is used for the sample having an ionic charge, or the sample is ionizable.
The stronger the opposite charge on the sample with respect to ionic change on the stationary phase, the stronger the attraction between sample ion and stationary phase; hence, the longer it will take longer to elute.
Here, the mobile phase is an aqueous buffer, where pH and ionic strength are adjusted to control elution time.
5. Bioaffinity chromatography or Affinity chromatography
Affinity chromatography is the most characteristic chromatographic method for separating a biomolecule from a mixture. The separation occurs based on a highly specific macromolecular binding interaction between the biomolecule and another substance.
These molecular interactions involve the participation of common molecular forces such as the Van der Waals interaction, dipole-dipole interaction, electrostatic interaction, hydrogen bond, and hydrophobic interaction. This process is used for the separation of biomolecules such as antigen and antibody, enzyme and inhibitor, hormone and carrier, receptor and ligand, or protein and nucleic acid.
This technique is highly specific and provides a high resolution of separation because of the fact that the two participating compounds are ideally suited to each other both spatially and electrostatically.
| Separation Principles | Interaction of substance with stationary phase | Example components that can be separated using the method |
| Normal Phase HPLC (NP HPLC) | Hydrophilicity (High Polarity) | Saccharides, Nuclear acids, etc. |
| Reverse Phase HPLC (RP-HPLC or RPC) | Hydrophobicity(Low Polarity) | Small molecule pharmaceuticals, Vitamins, etc. |
| Ion-Exchange HPLC | Electrostaticity charged Ions | Inorganic ions, Amino acids, Protein, etc. |
| Size-exclusion HPLC (SEC) or Gel Permeation or Filtration Chromatography | Smaller molecular size | Synthetic polymer, Biopolymer, Polysaccharide, etc. |
| Bioaffinity chromatography or Affinity chromatography | Highly specific macromolecular binding interaction between the biomolecule and another substance | Antigen and antibody, enzyme and inhibitor, hormone and carrier, receptor and ligand, or protein and nucleic acid. |
Instrumentation of High Performance Liquid Chromatography (HPLC) and application
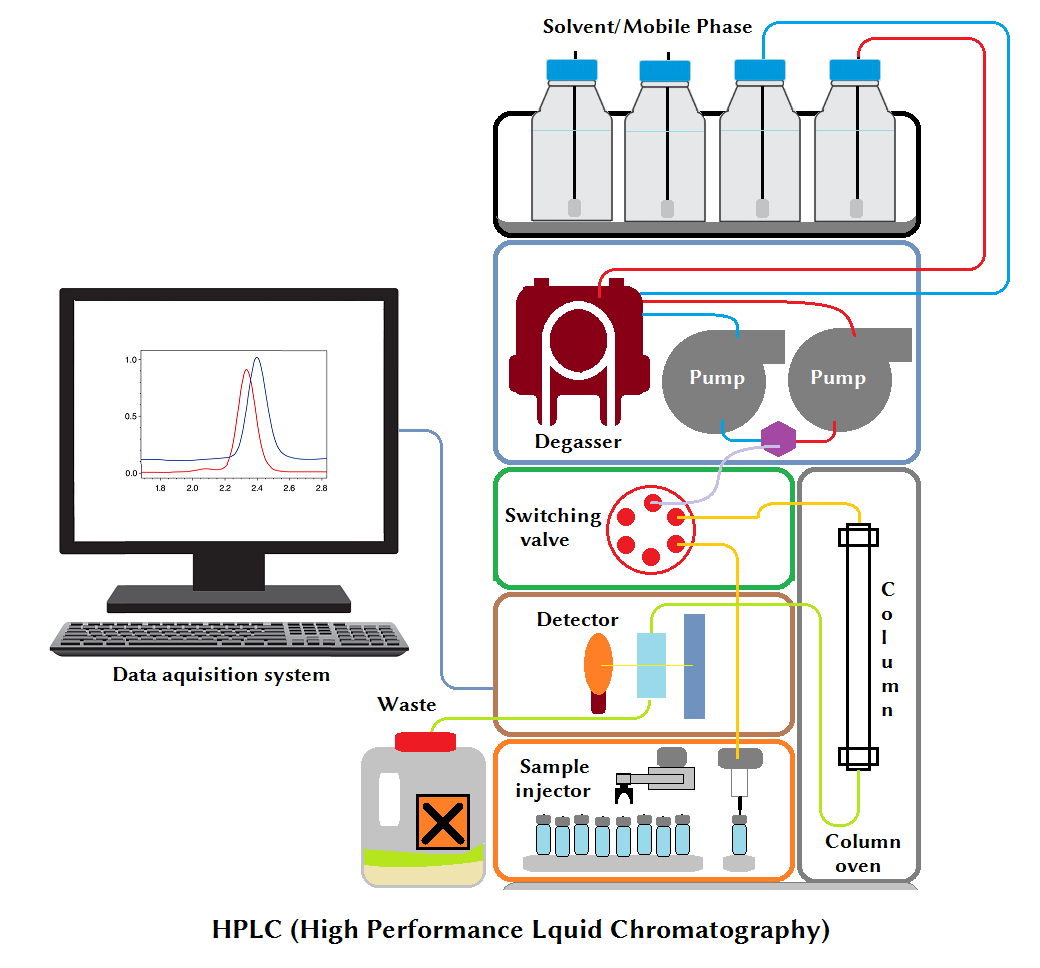
An HPLC instrument has at least the following elements shown in the schematic diagram of Instrumentation of High Performance Liquid Chromatography (HPLC).
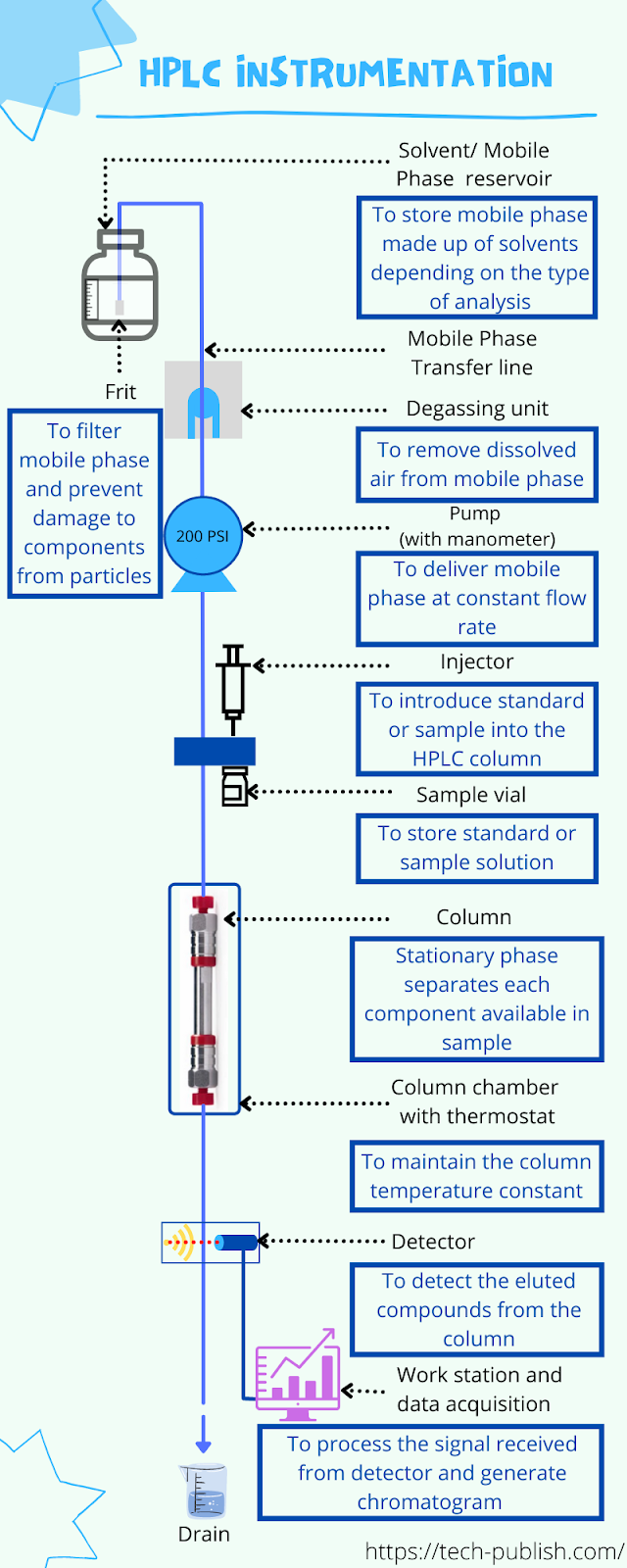
Typical instrumentation setup of HPLC includes the following:
- Mobile Phase or Solvent reservoir
- Mobile phase transfer line
- Frit (sinker frits in the mobile phase reservoirs)
- Degassing system
- High-pressure HPLC pump with manometer OR Solvent delivery system
- Sample injector
- Column
- Column Oven or Column chamber with thermostat
- Detector
- Data acquisition module
- Workstation to process acquired data
Lets understand HPLC instrumentation with detailed explanation of each component.
(a) Mobile Phase or Solvent reservoir
A Mobile Phase or Solvent reservoir holds the mobile phase or solvent. It is pumped through the system with the help of a mobile phase transfer line and high pressure pump. The mobile phase reservoirs are typically made up of glass covered with special caps.
Filter (Frit) and mobile phase transfer lines are used to connect the mobile phase reservoir to the HPLC instrument.
The number of Mobile Phase or Solvent reservoirs used for HPLC analysis is dependent on the type of chromatographic conditions required during the analysis. Examples of conditions are isocratic, gradient, etc.
(b) Mobile phase transfer line
Tubing with long length and small diameter, stainless steel/ polyether ether ketone (PEEK), or suitable capillary tubing is used to pump mobile phase through the HPLC system. Operating pressure for regular HPLCs ranges from 5800 – 7000 psi (400 – 500 bar), suitable for HPLC column hardware, whereas UPLC operates up to 15,000 psi.
Material of construction plays an important and vital role in the integrity of the system’s flow path. The material must be chemically inert and non-reactive with the sample and the mobile phase. Incompatibility of the tubing can cause samples to stick to the tubing surface, causing carryover, sample loss, or low yield in the case of preparative HPLC.
Capillary tubing bore must be smooth for limiting Newtonian flow through the sample loop. Accurate capillary tubing size and uniform inner surface yield accurate outcomes. The tubing should be free of contamination, such as grease, oil, and any other foreign material as part of the tubing manufacturing process or leftover from previous use.

The following three-point should be be considered for high-pressure tubing:
(i) Compatibility of tubing outer diameter (OD) with the receiving ports.
(ii) Inner diameter (ID) accuracy as per specific application. The smaller the inner diameter, the sounder the results.
(iii) Make sure the tubing is of the correct length for the application. The longer the tube, the higher the flow path volume. Higher flow volume may dilute the sample and could cause sample components to separate and merge back together.
Precautions while making tubing connections.
(i) Connection end should be burr-free and flat to fit tubing uniformly against the receiving port to prevent forming dead volume pockets.
(ii) Entering a tubing hole with a receiving port to reduce turbulence and backpressure.
(c) Frit (sinker frits in the mobile phase reservoirs)
It is essential to maintain mobile phase particulate free. Particulates in the mobile phase can cause trouble to the pump, injector or may cause damage to the column. As a general rule, mobile phase is filtered before use, however, sinker frits (5 to 10 micron pore size) should be attached at the end of inlet tubing that dips into the mobile phase reservoir.

The use of inlet frit has many advantages:
i. Providing extra protection against particulates contaminants entering the pump, injector, and column
ii. Holds the inlet line at the bottom of the mobile phase reservoir and prevents the tubing from creeping out of the reservoir. Therefore, inlet frits are often called “sinkers”. It helps keep the inlet tubing submerged in the mobile phase.
(d) Degassing system
When atmospheric air comes into contact with the solvent/ mobile phase, atmospheric air gets dissolved in the solvent/ mobile phase. As per Henry’s law…’the mass gas that dissolves in a liquid is directly proportional to that gas’s partial pressure above the liquid’.
Hence, all mobile solvents and mixtures, including a mixture with inorganic or organic compounds, contain more or less dissolved gasses. The proportion of air dissolution depends on the mobile phase composition, pressure, and temperature. For example, when the mobile phase is made up of organic solvent and aqueous liquid, both contribute an amount of dissolved air in the mobile phase. However, when the solubility of air is less than the available component in the mobile phase, the solution becomes supersaturated with air. This condition causes instability in the mobile phase, and air will bubble out from the solution.
The dissolved gasses generally consist of oxygen, carbon dioxide, and nitrogen. Their presence can cause adverse effects on the separation and also cause difficulty in evaluating the chromatograms.
The air bubble in the mobile phase will create several problems as follows:
| Air bubble in mobile phase at | Problem occurs |
| Pump | Malfunction of pump if it is vacuumized because of long suction distances or clogged intake fritsDisturbance in mobile phase flowFluctuation in flow rate of mobile phase |
| Column | Column efficiency decreases Fluctuation in retention time |
| Detector | Interference in detector signal causes erroneous results |
There are two main methods of gas removal from the mobile phase with the help of a degasser.
i. Helium sparging or purging: In this method, helium is bubbled through the mobile phase, which removes around 80% of dissolved gasses.
ii. Gas osmosis: The mobile phase is passed through a semi-permeable membrane in this gas removal method. This semi-permeable membrane is kept within the vacuum chamber. This semi-permeable membrane tube is permeable to gasses, but it does not permit liquids to pass through it. In this way, the dissolved gasses in the mobile phase diffuse across the membrane and into the vacuum chamber. The efficiency of this method is to remove more than 60% dissolved gasses.
The degassing method can be used separately or can also be combined.
(e) High-pressure HPLC pump with manometer OR Solvent delivery system
In simple column chromatography (open-column), the mobile phase passes through the column packing with the help of gravity, and the particle-size packings range from 50-100 micrometers. In this case, separation takes an extremely long time.
To improve the performance and for reducing the time required for separation, smaller particle size packings such as particle size 10 microns and below are used. In that case, passing the mobile phase through the column requires high pressure to pump it.
In the HPLC, the function of the pump is to maintain a constant flow of mobile phase regardless of resistance and back pressure because of column packing.
- Constant Pressure Pumps
- Constant Flow Pumps
- Positive displacement (Syringe) pumps
- Reciprocating Piston Pumps
- Single-Piston Reciprocating Pumps
- Dual-Piston Pumps
- Dual-Piston In-Parallel Pumps
- Dual-piston In-Series Pumps
- Dual piston (Different volume pistons)
- Dual piston (Different piston speeds)
- High-Pressure and Low-Pressure Mixing Systems
- High-Pressure Mixing Pumps
- Low-Pressure Mixing Pumps
1. Constant Pressure Pumps:
This technique of pressure pump is the most simple, inexpensive, easy to maintain, and easy to operate. In constant pressure pump design, pressure is generated using a gas cylinder. Gas, such as nitrogen pumps the mobile phase into a column by pressuring the mobile phase in the reservoir. In this system, the flow rate of the mobile phase is dependent on column resistance. In case of pressure drops, flow rate changes and directly impacts the retention time of the components to be separated. As a result, there is potential for gas solubility in the solvents.
The advantage of this system is that it provides pulse-less and continuous pressure with high flow rates. However, this system is not suitable for gradient elution. Also, the flow rates can change depending on the solvent viscosity change because of either temperature variation or change in composition.

2. Constant Flow Pumps
Constant-flow pumps systems are basically working using two basic principles: Positive displacement (syringe) pumps and Reciprocating pistons. The Constant flow rate pumps have been used since the 1960s, i.e., introducing the first commercial HPLC systems.
The constant flow rate approach is very important when it is used for analysis purposes. While performing an analysis, a detector signal is captured and plotted against with respect to the analyte’s retention times. The peak retention volume is equal to the retention time of the analyte multiplied by flow rate; it must remain constant during the entire chromatographic run to get adequate analysis results of chromatographic peak area versus time.
The primary advantages of these systems are their ability to get reproducible elution volume and peak area, irrespective of mobile phase viscosity or column blockages (Within the pressure limit of the HPLC pump).
The advantage of reciprocating piston pumps is that they can maintain mobile phase flow rate for as long as you want. However, in the case of a syringe pump, it requires refilling once it displaces its entire volume.
However, the syringe pump has the advantage that there is no pulsation flow and pressure, unlike the reciprocating pump.
This approach is useful in micro-HPLC applications where the syringe pump maintains a constant flow at a lower flow rate, such as a microliter per minute.
a. Positive displacement (Syringe) pumps
The Positive displacement (Syringe) pumps are generally helpful for precise constant flow without pulsation where there is a constant load. The syringe pump system can also be used to generate flow by using two or multiple syringes. Syringe pumps are mostly used for micro or nano HPLC instruments and portable HPLC systems. In such a system, the required flow rate is less. The compact pump design is possible using a syringe system.
The main disadvantage of Positive displacement (Syringe) pumps is that they require frequent refilling. The chromatographic separation stopped during the pump re-filled.
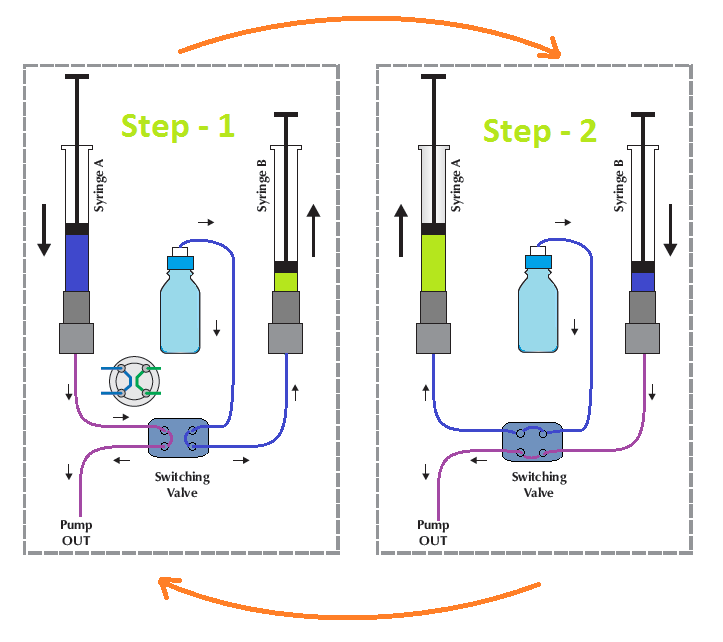
In the above schematic diagram, when Syringe A supplies its volume to the system, Syringe B is filled through the switching valve from the mobile phase reservoir. When Syringe A is emptied, the valve switches to Syringe B, which starts delivering its volume. Syringe A starts with its filling cycle, and the procedure is repeated again.
In this two syringe system, one syringe is always filled completely when the other end its delivery cycle. The delivery syringe starts a little bit earlier that is prior to the valve switches, so that it pre-compress the liquid for constant delivery.
b. Reciprocating Piston Pumps:
Generally, the HPLC uses the reciprocating piston type of pump design. The pumping process is driven by a stepper motor. The motor drives a rotating disc or cam that pulls the piston back and forth. During each pump stroke, a small amount of mobile phase is pumped. A flexible seal is used in the setup of piston design to prevent solvent leakage from the pump. Check valves are used in the pump to maintain pressure and a one-way mobile phase flow. Refer following schematic drawings to understand the principle.

The reciprocating pumps are further classified in various types based on number of pistons and its arrangements as follows:
i. Single-Piston Reciprocating Pumps
ii. Dual-Piston Reciprocating Pumps
1. Dual-Piston In-Parallel Pumps
2. Dual-piston In-Series Pumps
3. Dual piston (Different volume pistons)
4. Dual piston (Different piston speeds)
iii. High-Pressure and Low-Pressure Mixing Systems
1. High-Pressure Mixing Pumps
2. Low-Pressure Mixing Pumps
i. Single-Piston Reciprocating Pumps
The single-piston reciprocating pumps were used in early days of HPLC development. Schematic diagram of the single-piston reciprocating pump mechanism is shown above.
These pumps have following advantages:
- Lower cost
- Compact
- Continuous delivery
The weakness or disadvantages are:
- Pulsed flow because of piston recharge cycle
- Flow of the mobile phase gets intermittently stopped during the fill cycle and will not be not smooth. Refer to the following representation.

- Noisy baselines because of intermittent stoppage of flow
While using the HPLC for components analysis, the flow with a pulse is undesirable because it can cause detection issues, the possibility of erroneous quantitative analysis, and less column life because of column failure.
To overcome these limitations, the single-piston mechanism switched to the dual-piston pump in the early 80s.
ii. Dual-Piston Reciprocating Pumps
Dual-piston pumps are a widely used mechanism used for HPLC pumps nowadays. The Dual-Piston pomps are available with different configurations, viz. Dual-Piston In-Parallel Pumps, Dual-piston In-Series Pumps, Dual-piston with different volume, Dual-piston with Different piston speeds.
Each type of pump and its mechanisms are detailed below.
1. Dual-Piston In-Parallel Pumps
In dual-piston in-parallel pumps, two pistons are set at 180 degrees out of phase, and output of the mobile phase is combined from two heads. Both the pistons’ cams are driven by the same motor. With this dual pump approach, the flow of the mobile phase becomes smooth and less pulsing to the Liquid Chromatographic (LC) system.
In this mechanism, when one pump stroke takes the mobile phase, the mobile phase delivery simultaneously occurs from the second pump head. Therefore, using this system, when one pump cylinder is getting filled, the other delivers the mobile phase. Thereafter, when the second pump is getting refilled, the first one delivers the mobile phase.
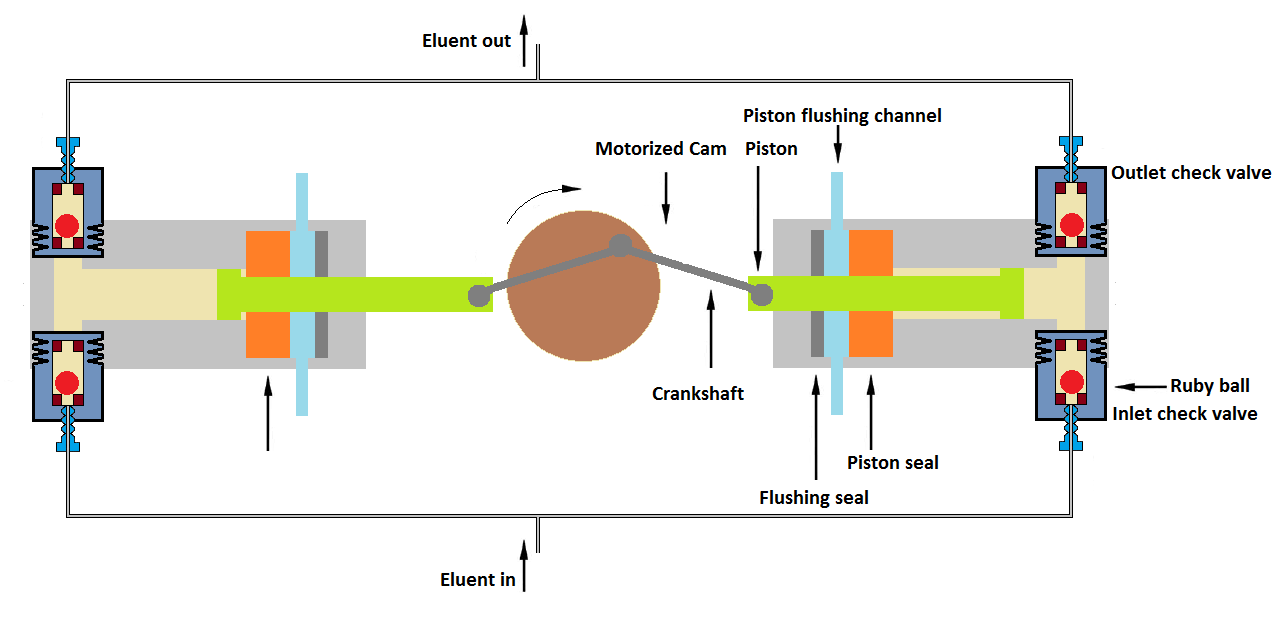
2. Dual-piston In-Series Pumps (both pistons have same volume)
Two pistons are set in series in dual-piston in-series pumps as per the following schematic diagram. In this mechanism, separate pistons’ cams are driven by the same or two separate motors.
In this pump design, the first piston delivers a mobile phase to the second piston. The piston movement is designed in such a way that the solvent is delivered from the first pump cylinder into the second pump cylinder without compression and creating pressure fluctuation. This is a very accurate mechanism with the minimum pulsation of flow.
Dual-piston In-Series Pumps are adopted by HPLC machine market leaders such as Agilent’s HPLC systems and Waters HPLC systems.
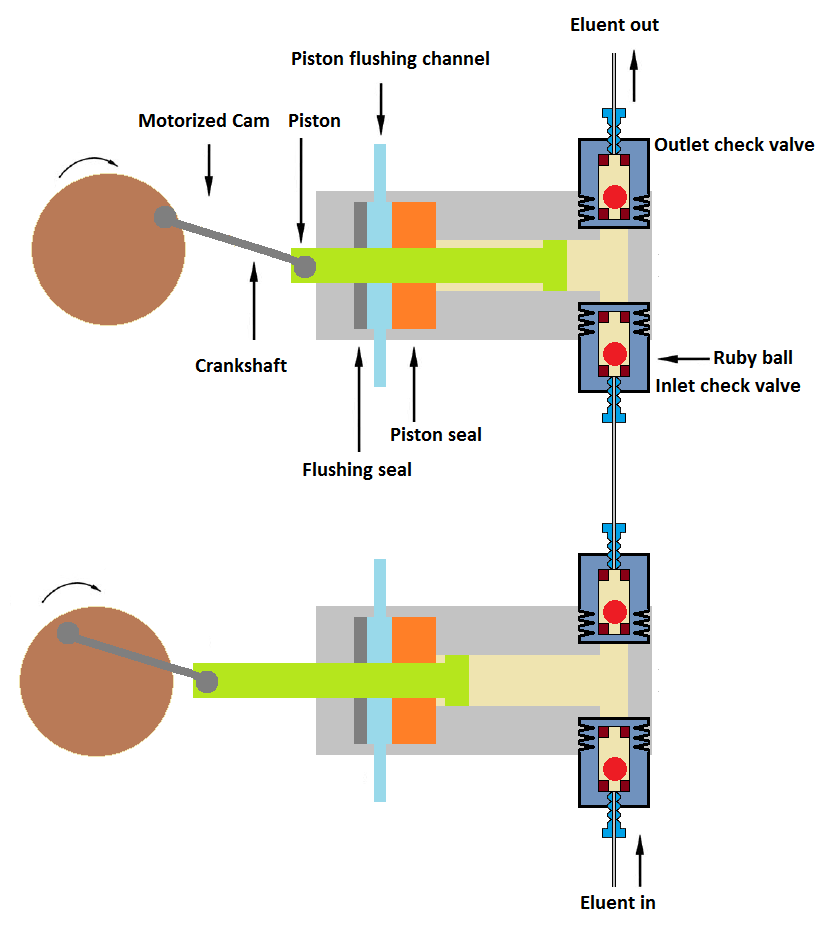
3. Dual piston (Different volume pistons)
In this mechanism, small volume pistons and large volume pistons are connected in series. The large volume piston intakes the mobile phase while the small volume piston pump pushes the mobile phase-out. A large volume piston fills the smaller piston-cylinder simultaneously when it is discharging and dispenses the mobile phase into the LC system.
This mechanism has advantages of pressure and pulse-free delivery of the mobile phase into LC.

4. Dual piston (Different piston speeds)
In this mechanism of the HPLC pump, the piston size is the same, but the speeds of both pistons are different. Eluent is received in the mixing chamber by first low speed (around 1mL/ min) piston pump, and it is transferred into the delivery chamber via transfer line at high-speed piston pump (around 100 ml/min). The mechanism provides high-efficiency mobile phase mixing because of higher turbulence in the delivery chamber.
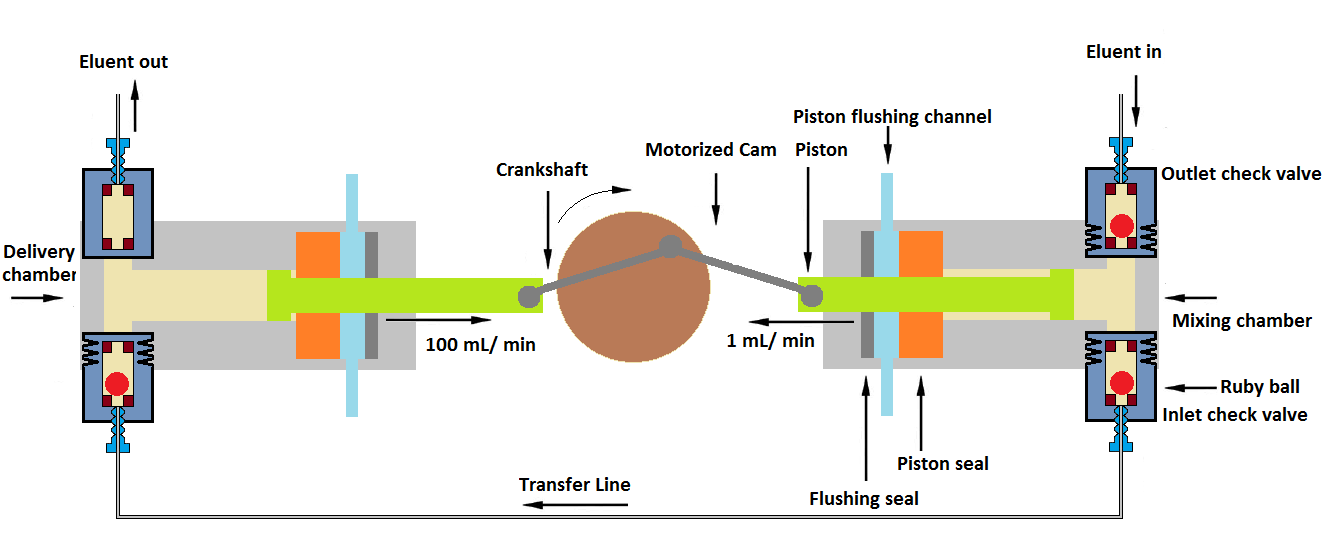
This system has the advantage of eliminating air bubbles and cavitation. This mechanism also prevents backflow while mobile phase delivery and without pressure pulsations.
iii. High-Pressure and Low-Pressure Mixing Systems
Mobile phase mixing systems are categorized into two types based on the design. One is high-pressure mixing (also called as binary pumps) and low-pressure mixing systems (also called quaternary pumps).
1. High-Pressure Mixing Pumps
High-pressure mixing systems consist of two or three pressure pumps for conveying different solvents. In this system, separate pumps deliver solvent to the mixing chamber. In this chamber, a mixing of solvents occurs with higher pressure (at the high-pressure side).
Therefore, the design is called a high-pressure gradient system. From the mixing chamber, the blend of mobile phase reaches the column. Electronic is controlling the system to ensure the consistent and constant flow of volume. To deliver each solvent, separate pumps are required; hence, this system is comparatively more expensive than a low-pressure system.
However, the advantage is a smaller dwell volume. The dwell (or system) volume is the volume measured within the HPLC system from the point where the mobile phase begins to mix to the entrance of the column.
The advantages of this mixing system are:
- It has precise, reproducible, and better composition accuracy up to 0.1%.
- Lower dwell time enables the system to deliver changes in the gradient rapidly to the column, hence, faster re-equilibration between two sample runs
2. Low-Pressure Mixing Pumps
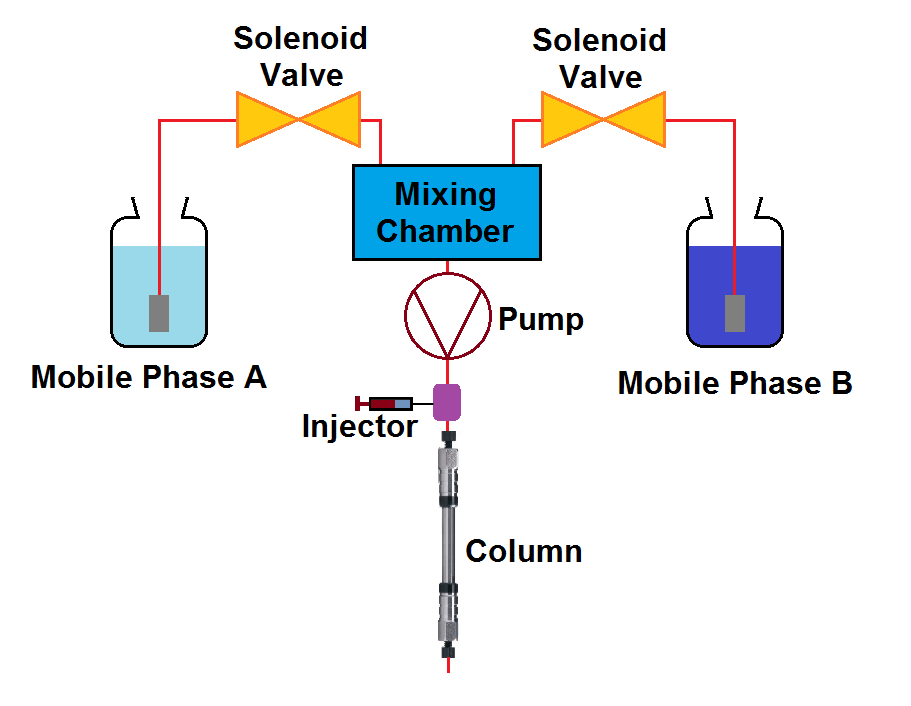
Low-pressure mixing systems consist of two or more mobile phase reservoirs connected with a solenoid valve (proportioning valve) which is further connected with a mixing chamber. Valves can be controlled so that they can provide the desired composition of the mobile phase in the mixing chamber.
Mixing of the mobile phase occurs on the low-pressure side prior to entering the pump; hence, it is called a Low-pressure mixing system. The mechanism is capable of delivering mobile phases up to 4 different combinations.
Low-pressure systems are comparatively less expensive. Beneficial for method development projects because of the possibility to use quaternary systems for operation.
Disadvantages of low pressure system are
- Dwell volume is higher
- Potential for air bubbles in the system, hence, a degassing system is necessary for such systems
- Less compositional accuracy hence less retention time precision
(f) Sample injector
Sample feed is one of the critical aspects of HPLC. A sample injector is a device used to inject sample solutions into the system. The function of the injector is to inject or load the sample into the HPLC column.
There are the following types of sample injectors typically used in the HPLC.
i. Rheodyne or loop injector
ii. Septum Injector
iii. Stop flow Injector
iv. Autosampler
While using the sample injector, following characteristics are significant and critical to be considered:
- The injector should be able to load a consistent and precise amount of samples during each sample run.
- While injecting the sample in to the HPLC column, there should not be any pressure fluctuation or disturbance in the system.
- It should not pop out the air bubble while loading the sample.
- Should be able to withstand at high pressure (6000 – 22,000 psi)
- Should be compatible with commonly used HPLC solvents and mobile phase components
- No or very low carryover
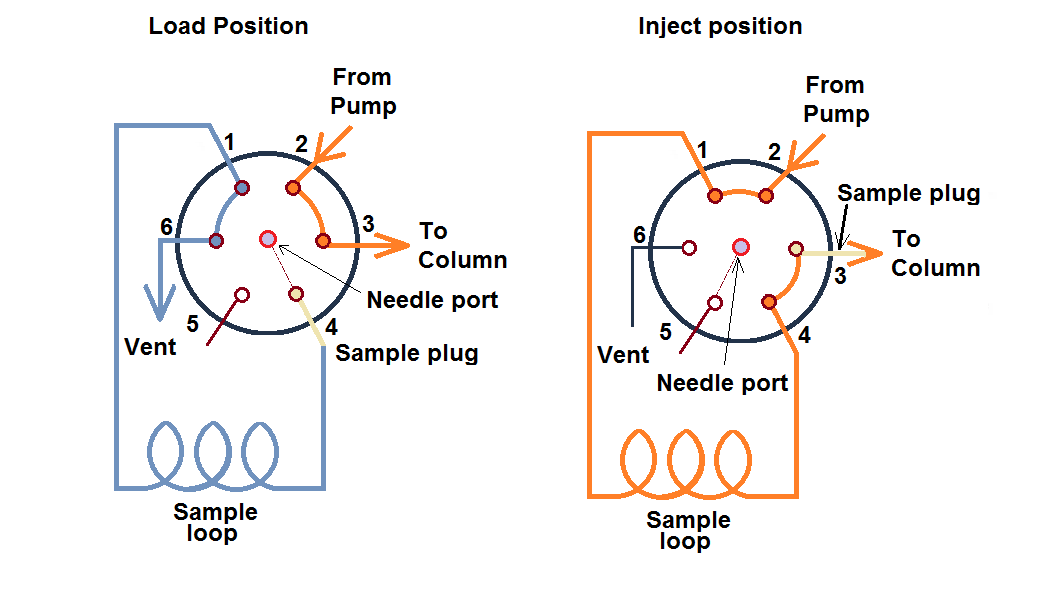
i. Rheodyne or loop injector
This is a manual sample injector placed in the market by an organization, Rheodyne Corporation. This injector has a six-port valve system and two positions. The first position is the load position and the second position is inject position.
In this type of injector, sample volume loop is interchangeable from for example, 6 µL to 2 mL. To inject the sample manually, a 22 gauge needle is used with a blunt tip.
Once the sample is injected at load position, the injector is manually rotated to set the inject position. This system operates in such a way that it does not create air bubbles and does not disturb the system the pressure and flow rate.
ii. Septum Injector
A septum type injector consists of a rubber septum through which a needle is inserted to inject the sample. Septum acts as a seal of an injector port. Septum must withstand high pressure generated in the system.

iii. Stop flow Injector
In this type of injector, the flow of the mobile phase stops when a sample is injected. Because of the mechanism of stop flow, a ghost peak is generated in this type of injector.
iv. Autosampler – Types and Operating Principles
Auto sampler has following main components
- Sampling needle
- Injection valve
- Sample loop
- Sample compartment
- Metering device
For different types of autosampler, operating principles are different. There are three key principles based on functional differences. Those are as follows:
- The Pushed loop design or Push-to-fill autosampler design
- Pulled-loop or Pull-to-fill autosampler design
- Split-Loop Design, Integrated-loop, Flow-through needle, or Needle-in-loop autosampler design
a. The Pushed loop design or Push-to-fill autosampler design
The autosampler design of Pushed-Loop or Push to Fill is similar to the manual injection system. The first step is puncturing the septum of the sample vial using a needle and collecting the sample by pulling the required volume. Then the sample is moved to the injection valve and inserted into a low-pressure connector.
The sample is pushed into the sample loop with the help of the syringe mechanism. Lastly, the injection valve is rotated to achieve the inject position so that the mobile phase flow from the pump to the column is directed through the sample loop, and the sample is injected into the column.
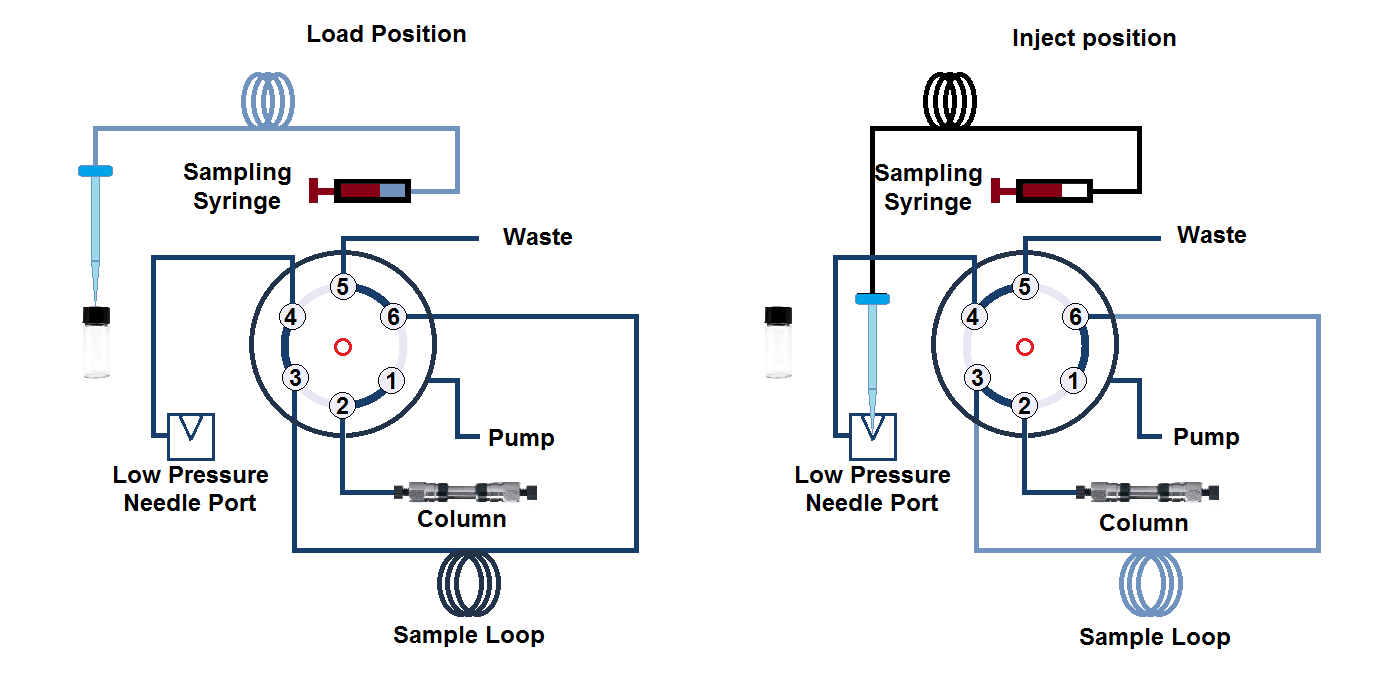
b. Pulled-loop or Pull-to-fill autosampler design
The name of the Pulled-loop or Pull-to-fill autosampler design is self-explanatory based on its design. In this design, the sample is collected into the sample loop with the help of syringe suction while injector in the load position.
Once the loop is filled, the sampler position is changed to inject position to deliver the sample aliquot to the HPLC column.
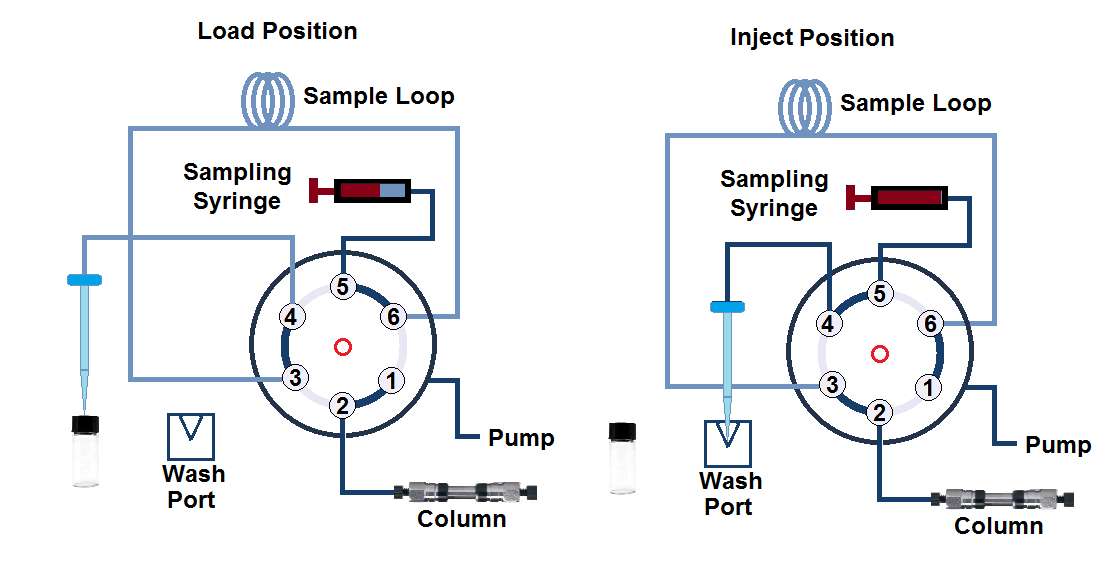
c. Split-Loop Design, Integrated-loop, Flow-through needle, or Needle-in-loop autosampler design
In this design of autosampler, the sampling needle is a part of the sample loop. This setup consists of high-pressure needle seals. While the autosampler is in the load position, the sample needle moves to the vial and splits the loop into two portions. There are two methods of sample loading.
(I) the First approach is the use of a Flow-through high-pressure metering device. This approach has the advantage that it is more precise and provides constant flushing of the device to prevent the accumulation of air bubbles.
(II) In the second approach of sample loading, the metering device (sampling syringe) is placed outside the high-pressure flow path at the end. In this design, the sampling syringe does not get flushed throughout the run.

Following are the Features of (i) The Pushed loop design or Push-to-fill autosampler design, (ii) Pulled-loop or Pull-to-fill autosampler design, and (iii) Split-Loop Design, Integrated-loop, Flow-through needle, or Needle-in-loop.
| Features | The Pushed loop design or Push-to-fill autosampler design | Pulled-loop or Pull-to-fill autosampler design | Split-Loop Design, Integrated-loop, Flow-through needle, or Needle-in-loop autosampler design |
| Mechanical simplicity | Very simple design | Medium simple | Complex |
| Ease of flushing | No | Very easy to flush | Very easy to flush |
| Carryover | Low | Low | Very low |
| Impact on dwell volume | Less | Medium | Less |
| Conserves sample/ Consumption of sample | Low conservation, high consumption | Medium conservation, medium consumption | High conservation, low consumption |
| Variable injection volume/ Injection volume flexibility | Low flexibility | High flexibility (different size loop required) | Very High flexibility (only one sample loop require) |
| Needle seal problems | Less issues | Medium | High issue |
| Precision of injection volume | High | High | Very high |
(g) Types of HPLC columns
In chromatography, HPLC columns play a crucial role in separating components from the solution.
HPLC column is hardware that acts as a stationary phase in the HPLC system. It consists of a tube containing chromatographic packing material and closed from both ends with fittings to facilitate connection with the HPLC system.
Important characteristics of (High Performance Liquid Chromatography) HPLC columns are:
- Leak free
- Consumes minimum volume
- No dead volume
- Chemically inert to mobile phase and sample
- Withstand at high pressure
Different Types of HPLC Columns Used in Analysis
- Types of HPLC Column based on Material of Construction
- Stainless steel Columns
- PEEK (Poly Ether Ether Ketone) Columns
- Glass Columns
- Types of HPLC Column based on Scale of Operation
- Analytical
- Semi-preparative
- Preparative
- Process/ industrial production
- Types of HPLC Columns based on separation technique
- Separation based on polarity or solubility
- Normal Phase
- Reverse Phase
- Separations Based on Charge or Ion-Exchange Chromatography
- Separations Based on Size (Size-Exclusion Chromatography or Gel-Permeation Chromatography)
- Separation based on polarity or solubility
Types of HPLC Columns based on Material of construction of column tube are as follows:
- Stainless steel: Most HPLC columns are constructed with this material as it has the advantage that it can withstand with higher pressure

Image source: orochem.com
- PEEK (Poly Ether Ether Ketone): PEEK is an engineered plastic. This material is semi-crystalline thermoplastic.
- It has extraordinary properties of mechanical and chemical resistance with high temperatures. It can be operated at a temperature of 250 °C or 482 °F). Also, it is a highly resistant material to attack by organic and aqueous environments.

Image source: www.idex-hs.com
To increase the strength, the stainless steel HPLC columns are lined with PEEK material to get an advantage of PEEK material property and strength of Stainless Steel.

The PEEK column is useful while handling compounds containing phosphate groups. Analyte having a phosphate group creates a phosphate-iron complex, and this complex has the potential to impact the peak shape resulting in low, precise quantitative analysis results. In such cases, metal-free PEEK columns help to improve chromatographic results with perfect peak shape for chromatographic applications.
- Glass: Less popular and less pressure tolerant. However, glass HPLC columns are used when inert surfaces are the most important characteristic that is required for special chemical or biological applications.

Image source: www.analytics-shop.com
The selection of HPLC columns are dependent on the various aspects:
- Scale of separation
- Analytical
- Preparative
- Process
- Separation techniques
- Chemical and Physical attribute
Following the flow chart helps select the column based on various aspects, such as column chemistry, physical property, and separation scale. Refer to HPLC Column selection/ classification chart.
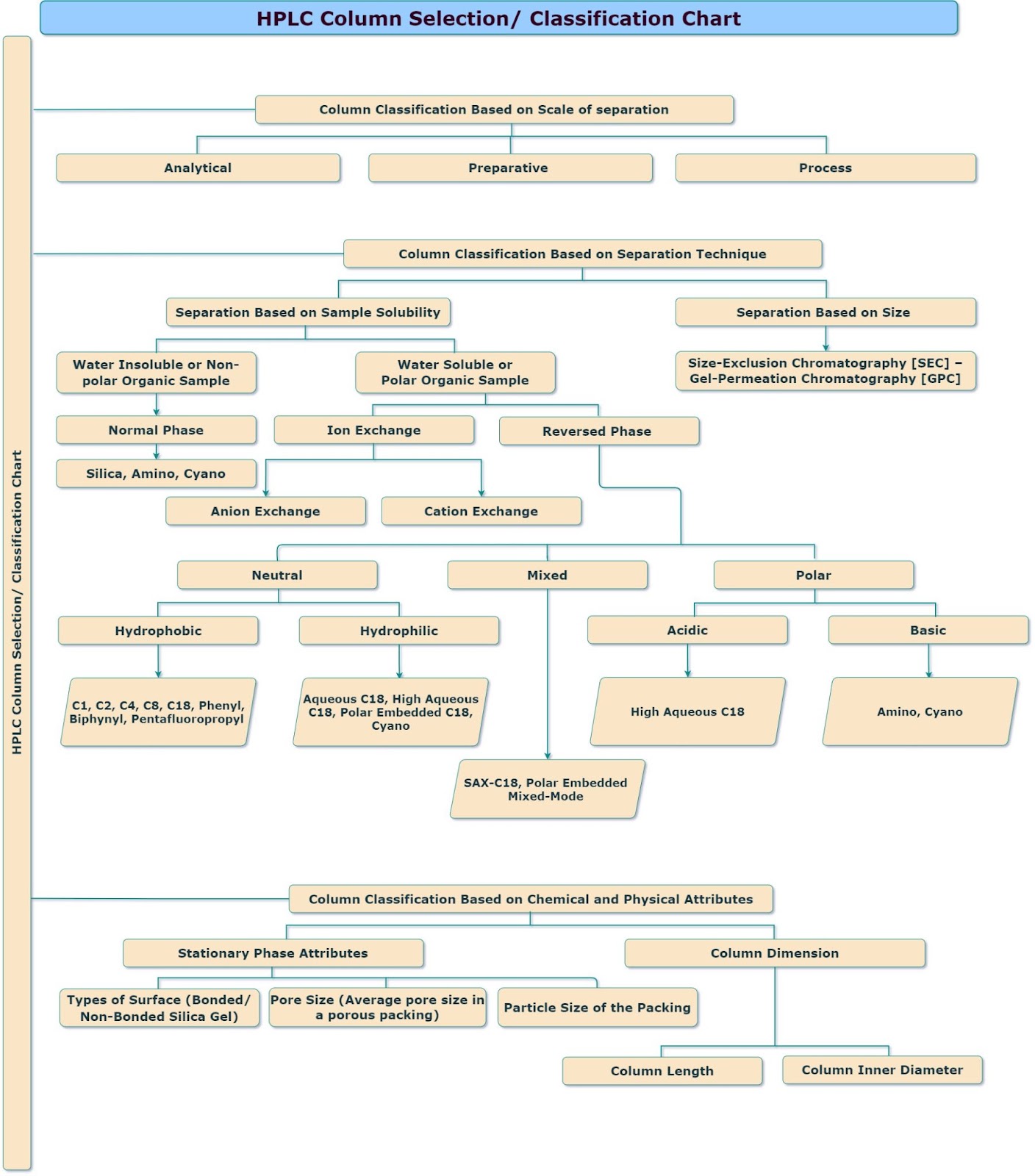
HPLC Columns selection and classification based on scale of separation:
HPLC is used for various purposes, such as identifying and qualifying the amount of compound in the solution, purifying the compound, and separating the specific compound from the mixture as part of the pure compound manufacturing process.
Based on the above criteria, column selections are made depending on the scale of operation. Those criteria are as follows:
| HPLC Column Classification based on scale of operation | Scale and Objective of HPLC columns | HPLC Column diameter | Particle size of HPLC column |
| Analytical | Just for identification and quantitative analysis | 1-8 mm | 1.7 to 10 micron |
| Semi-preparative | Purification of compound with less than 0.5 gram | 10-40 mm | 5 to 15 micron |
| Preparative | Compound to be purified in grams greater than 0.5 gram but not in kilogram | 50-100 mm | 15 to 100 micron |
| Process/ industrial production | Manufacturing quantity from grams to kilogram | More than 100 mm | More than 100 micron |

Schematic representation of Analytical and Preparative HPLC column
HPLC Columns selection and classification based on separation technique:
In principle, there are three primary characteristics of compounds that can be the basis for separations in the HPLC method. The characteristics are Polarity (Solubility), Electrical Charge, and size (Molecular Size).
Separation based on polarity or solubility:
The separation technique based on the polarity or solubility is mainly divided into two categories, normal phase chromatography, and reversed-phase chromatography.
The compounds with high dipole moments, such as water, are polar compounds. An aromatic compound such as benzene is a non-polar compound.
Compounds with similar polarity are attracted towards each other, and it is inversely proportional when dissimilar polarity exists and exhibits weaker attraction. Degrees of polarity-based attraction are the basis for chromatographic separation.
The following table shows the polarity of compounds based on their possession of functional groups.
| Hight Polar Molecule |
| Salts |
| Acids |
| Alcohols |
| Ketones |
| Eithers |
| Halogenated hydrocarbon |
| Aromatic hydrocarbon |
| Aliphatic hydrocarbons |
| Fluorinated Hydrocarbon |
| High Non-Polar Molecules |
In the polarity-based chromatography separation, the mobile phase and stationary phase are selected to create competition among the various compounds of the sample. Compounds with the similar polarity of stationary phase will elute last as it has strong attraction between them. On the other hand, compounds with similar polarity with the mobile phase will elute faster.
The chromatographic separation based on the polarity is further classified depending on the mobile phase and stationary phase combination.
| Chromatographic separation mode | Stationary Phase | Mobile Phase |
| Normal Phase | Polar | Non-polar |
| Reverse Phase | Non-polar | Polar |
Separations Based on Charge or Ion-Exchange Chromatography
The principle of separation in Ion Exchange Chromatography is the function of charged ions. In the case of Ion Exchange, the rule of attraction is the reverse of polarity-based separation. Like to like ions repel and opposite attracts. The strength of attraction is dependent on the acidic or basic functions on the surfaces of the stationary phase and compound.
In the case of cation exchange chromatography, it retains and separates +ve ions on a -ve surface.
Whereas, in the case of anion exchange chromatography, it retains and separates -ve ions on a +ve surface.
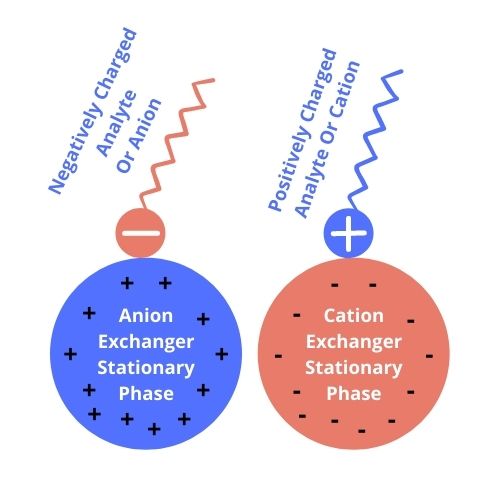
Characteristic of strong ion exchangers
- Functional groups are always ionized.
- Used to retain and separate weak ions.
- Weak ions are eluted by displacing the mobile phase containing strong ions that have an attraction towards the stationary phase.
- Weak ions are retained in the column. It gets neutralized by altering the pH of the mobile phase. This action loses its attraction and gets eluted.
Characteristic of weak ion exchangers
- Neutralized at specific pH value and loses its ability to retain ions based on its charge.
- On achieving charge, it retains and separates strong ions.
- To facilitate elution, the displacement method is used. Stationary phase exchanges are neutralized; hence, no attraction exists in the system. This condition permits elution of the analytes.
Separations Based on Size or Size-Exclusion Chromatography or Gel-Permeation Chromatography
Size Exclusion Chromatography is also called as Gel Permeation Chromatography or Gel Filtration Chromatography. In this separation technique, a smaller molecule of the compound in the solution diffuses dipper into the stationary phase matrix. Because of this, sampler molecule elution is impaired, and larger molecules elute faster.
This technique is generally useful for macromolecules characterization. Macromolecules include natural polymers, synthetic polymers, proteins, RNA, or DNA.
Schematic illustration of Size-Exclusion Chromatography is provided below. It provides clarity on how the Size or Size-Exclusion Chromatography works. From the mixture of a compound with a different molecular size, a compound with a higher molecular size (Compound B) is eluting faster than smaller molecular size (Compound A), and the solvent is used to mix the compound elutes at the end.
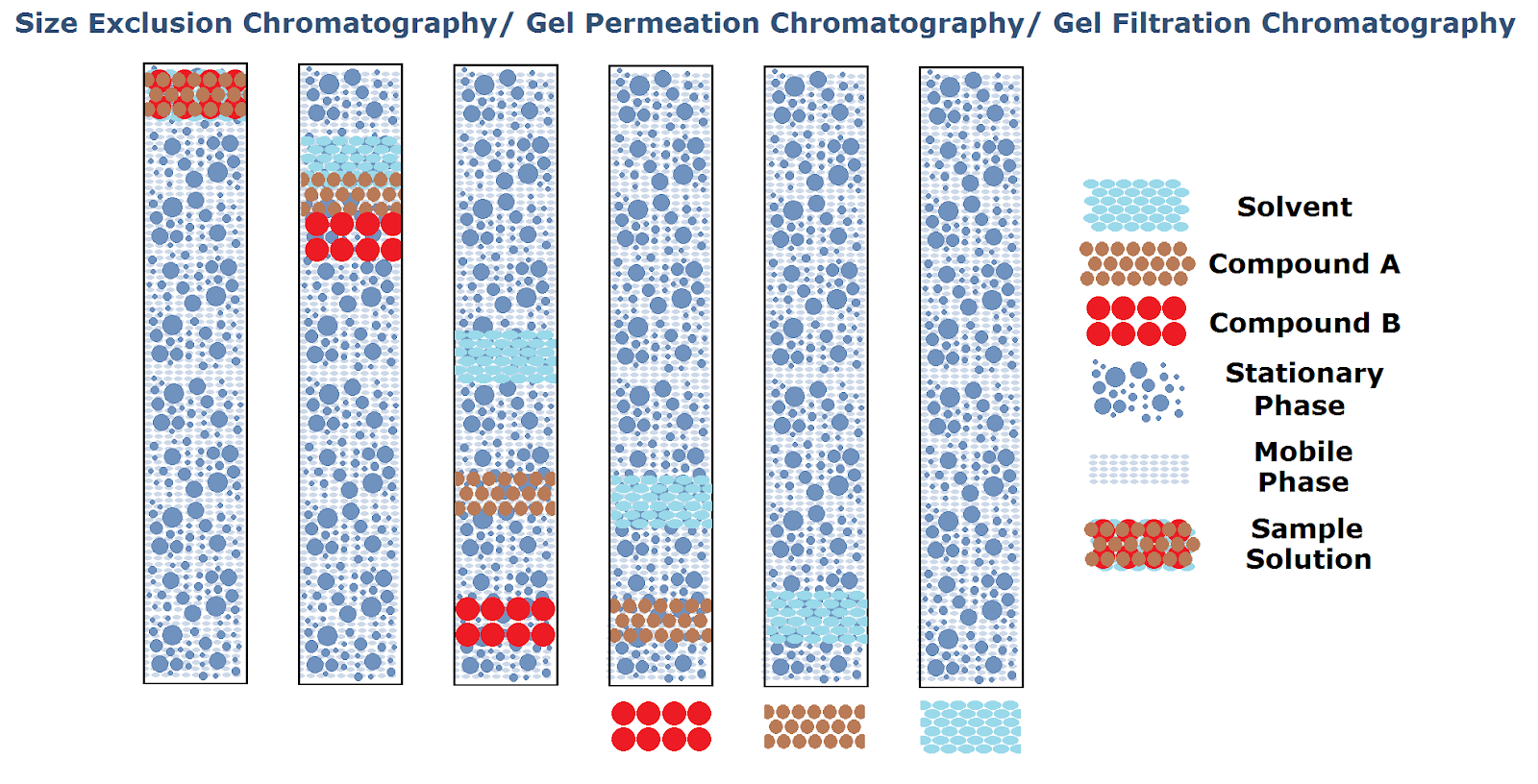
HPLC Columns selection and classification based chemical and physical attributes:
Chemical and physical attributes are important factors to consider while selecting the column.
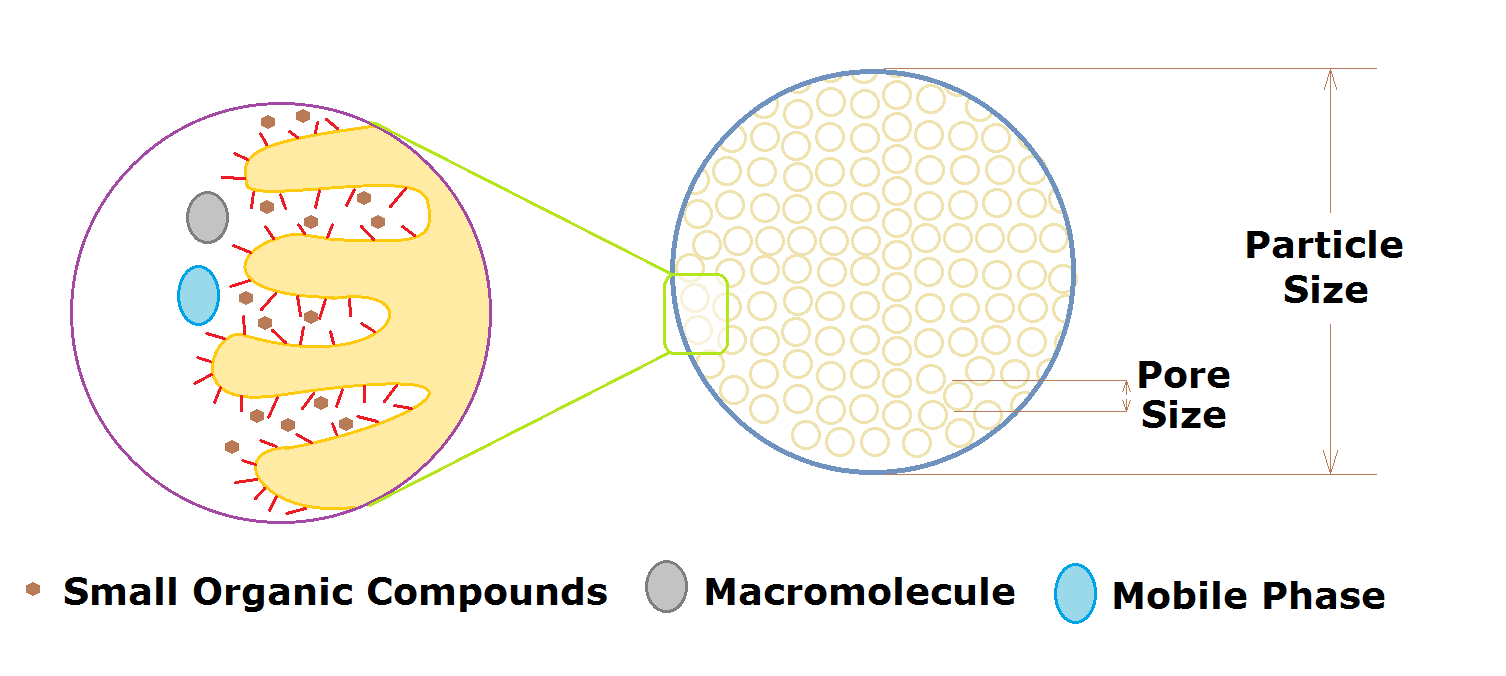
There are two key elements that determine the separation power or resolution which is achieved by HPLC columns are:
(i) Chemical attributes: Type of surface, surface bonding, and pore size of the stationary phase. This attribute helps to achieve selectivity. The selectivity (chemical separation efficiency) is mainly determined based on a combination of stationary phase and mobile phase.
(ii) Physical/ Mechanical attributes: Column length, particle size, and inner diameter. This attribute helps to achieve efficiency, which is measured by mechanical separation.
Significance of type of surface and surface bonding of stationary phase: Type of surface and surface bonding defines the column’s characteristic, such as the polarity of stationary phase (it decides Normal Phase Chromatography or Reverse Phase Chromatography) or change on the stationary phase (Ion exchange chromatography). These topics are discussed in detail in respective sections.
Significance of Pore Size of stationary phase:
Pore size is important in column packing because it provides the path to the molecules and allows molecules to interact with the stationary phase. It is an average size of pore in the packing material. The unit of measurement of pore size is angstroms.
| Pore size of packing material | Molecular weights |
| 80 to 120Å | Up to a molecular weight of 2000 |
| 200-450 Å | Over 2000 |
| 1,000Å and 4,000Å | Very high molecular weight proteins and vaccines |
Significance of Particle Size of stationary phase:
The claimed particle size of column packing is an average of claimed size. It generally gets distributed within ± 10% of the claimed size. For example, the claimed 10 µm size is dispersed between 9 and 11 µm. The particle size of column packing material varies from 5 µm to 1.8 µm. The efficiency of the column is measured using plate number or theoretical plate.
The smaller particle size of packing material in the column provides higher efficiency and has higher backpressure.
When the particle size of a column is decreased by half, the plate number/ theoretical plate count doubles (when column length and internal diameter of the column remain the same in both cases), and column backpressure increases to four times.

Significance of Column Length:
By keeping constant particle size of column packing, if column length is increased, it will have mechanical separation power. However, it increases the run time of analysis, increases the solvent consumption, and generates higher backpressure. Opposite of this, when column length is decreased, it reduces the analysis time but also reduces mechanical separation power.

Significance of Column Inner Diameter:
When a sample is injected into a lower internal diameter column, the peak goes higher than the comparative larger internal diameter. That means, when column diameter is decreased by half, the sensitivity will increase by four to five times higher (when injection mass remains constraint).
Low internal diameter column has the advantage of low-volume consumption, linear velocity, and increased column efficiency. Also, the backpressure, theoretical plates, and analysis time are not significantly affected as an outcome.
(h) HPLC Column Oven or HPLC Column chamber with thermostat
The use of a column heater or Column chamber with a thermostat helps improve performance and reduce the analysis time. The elevated temperature of the HPLC column helps in a faster chromatographic separation process and improves efficiency.
However, it has limitations that a mixture of compounds may co-elute if the temperature is not adequately controlled.
When column temperature increases, the retention time decreases to 1–2% for each 1 °C increment. Increased temperature is helpful in chromatography when the aim is to reduce the run time and reduce column backpressure.
The back pressure of the system gets reduced with an increase in column temperature. This happens because the viscosity of the mobile phase decreases, resulting in a decrease in flow resistance in the column.
The most famous mechanisms of column heaters are (i) block heater and (ii) air bath.
Block heater: In this type of heating mechanism, the column is directly in contact with the heat source (heating block). The heat transfer happens in this case through thermal conduction. The heating block consists of flexible heating tape or grooved metal block.
This mechanism provides precise and efficient real-time column heating.
Air bath: Air batch works on the principle of air convection with the help of circulating air systems. Since air is not a good, bad heat conductor, it is less effective in controlling the column temperature in real-time. Therefore, it takes more time to increase temperature than with a block heater.
In this type of heater, the heating of the column is controllable using the fan speed of the forced air thermostat.
Column heaters come with various ranges from 15 °C to 130 °C. Low-temperature column compartments are helpful for analysis of thermolabile materials.
Whenever a column oven is used to alleviate the column temperature, preheating of the mobile phase plays an important role because there is a change of cooling of the column wall through the mobile phase. Subsequently, it starts reheating at a slow rate with the help of a column heater. It again depends on the flow rate of the mobile phase. In such a situation, effective results of the use of the column heater are not achieved because there is a high chance that separation occurs at mobile phase temperature rather than the temperature set for the column heater. To rule out these possibilities, preheaters are used that preheat mobile phase to effectively use column heaters.
(i) HPLC Detector
The HPLC detector is an element of a chromatographic system that recognizes a substance that is eluted from the HPLC column by monitoring the change in mobile phase composition and converting it into an electric signal. The electronic signal is converted to a human-readable response with the help of software.
The characteristic of ideal HPLC detectors are as follows:
(a) Sensitive to all eluted substance and record it;
(b) Able to detect the eluted substance (able to generate a response of substance of interest);
(c) Not sensitive to temperature (should not be affected by changes in temperature);
(d) Should not be sensitive to mobile phase composition to facilitate in gradient elution;
(e) Should be able to detect minor changes in the concentration of analyte and provide a linear response;
(f) quickly transmit the signal;
(v) Cost-effective.
There is no HPLC detector that can act as a universal detector that can analyze all compounds; hence, depending on the compound characteristic and detection capability, a selection of detectors is made. Following are the examples of commonly used detectors used for liquid chromatography.
(i) UV-Visible HPLC Detector:
UV-Visible HPLC detector works on the principle of absorbance. This is the most commonly and widely used detector with a good sensitivity wide linear range. This HPLC detector is not sensitive to temperature fluctuations and gradient elution.
The commonly used source for UV detectors for HPLC is a low-pressure arc discharge deuterium lamp (Provides wavelength from 190 to 600 nm). The tungsten source is also used to provide a visible range from 400 nm to 950 nm.
In the UV-Visible type of HPLC detector, absorption occurs at a wavelength from 200 nm to 800 nm. The UV-Visible detector is sensitive to a molecule with (a) a double bond adjacent to an atom with a lone electron pair, (b) carbonyl group, (c) nitro group, (d) double bonds, (e) aromatic ring, (f) molecule has bromine, iodine or sulfur group and (g) molecule with inorganic ions such as Br, I, NO3, NO2
While using a HPLC UV-Visible detector, the mobile phase would be considered to have optical transparency in the UV-Visible range. This means that when the mobile phase passes through the detector, it should not provide any absorbance.
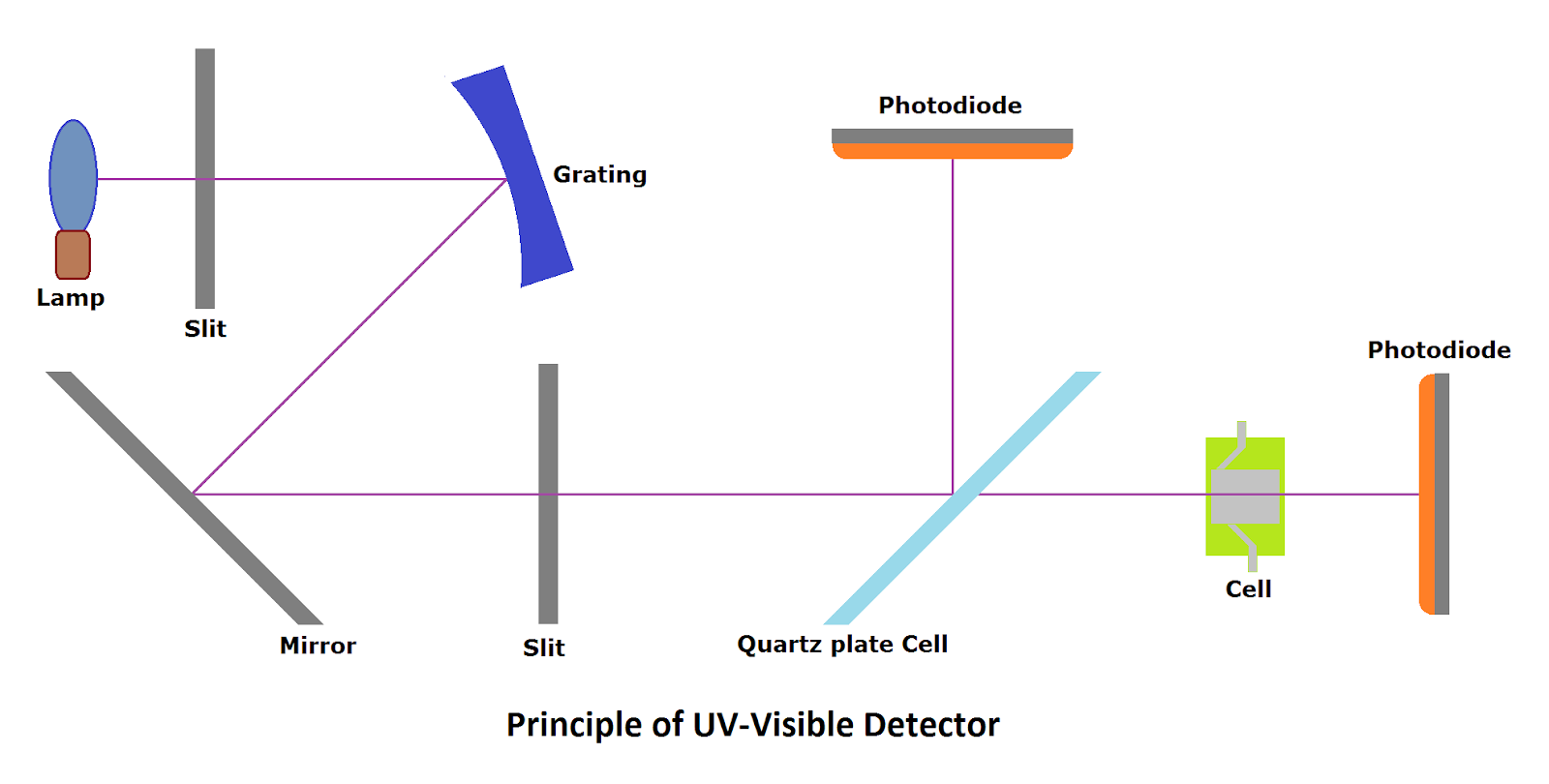
The sample passes through a clear colorless glass cell (flow cell) in the HPLC system. The UV-Visible light passes through the flow cell, and the sample absorbs a part of the light of the selected wavelength and gives a signal. The same sample will provide different absorbances at different wavelengths. The wavelength to be selected is determined during the method development phase.
Generally used, its wavelength is 254 nm. While a visible HPLC detector is used, it uses longer wavelengths from 400 to 700 nm.
(ii) PDA Detectors (Diode Array Detector or Photo Array Diode Detector) for HPLC :
In Diode Array Detector or Photo Array Diode Detector, full light first goes through the cell and reaches the polychromator (also called grating). This light then reaches a large number of the diode array. The diode array is very sensitive. Each diode receives a fraction of the information, converts it into the signal, and gets processed.
The advantage of the PDA detector is that it scans an entire spectrum at a time. Conventional UV-Visible detector scans samples in two dimensions: time and sensitivity, whereas PDA detectors scan the sample in three dimensions. The third dimension is wavelength in addition to time and sensitivity.
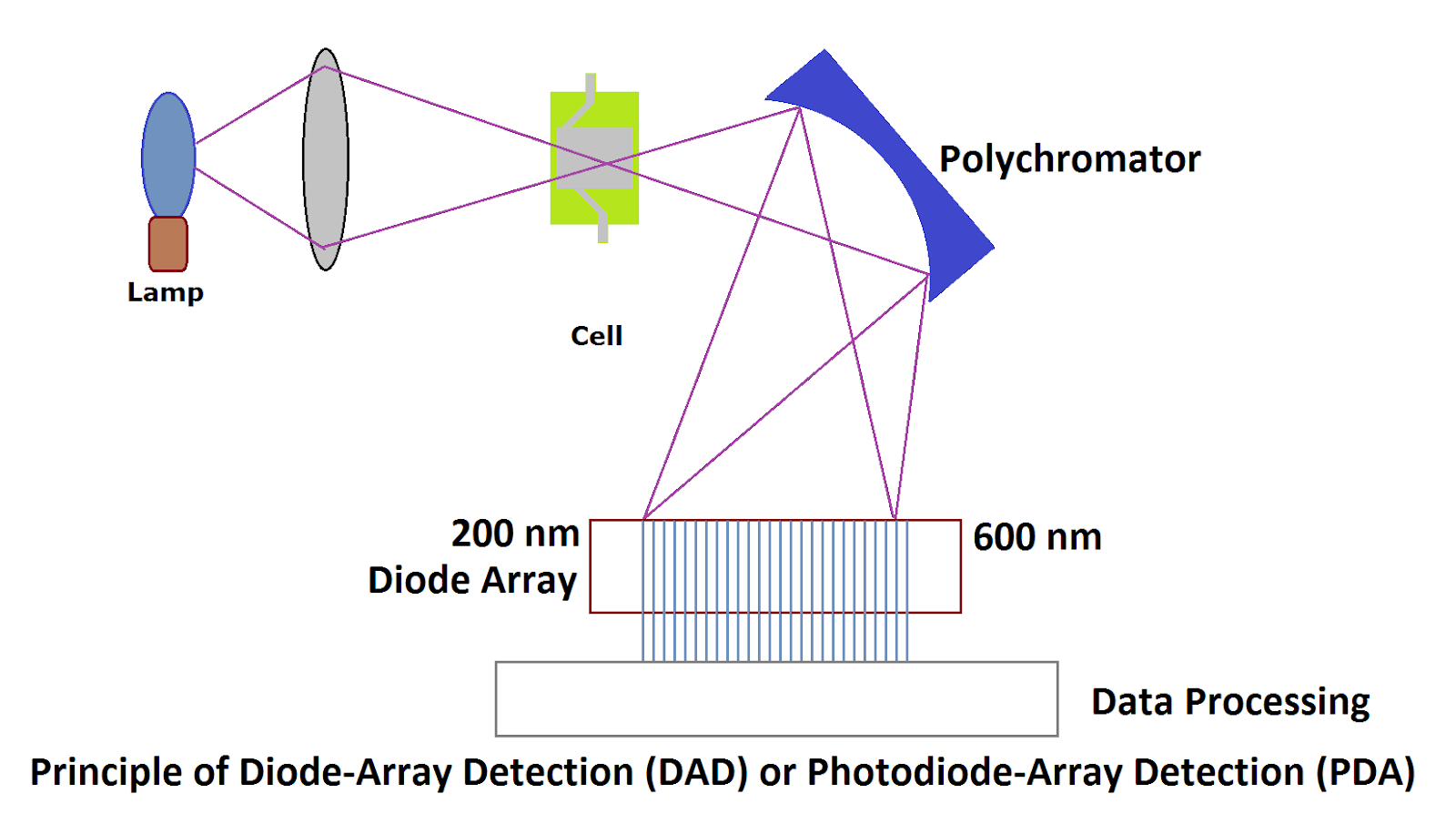
Advantages:
- HPLC PDA detector captures individual peaks for an entire range of wavelengths, and this process gets completed in a fraction of seconds.
- This allows us to compare the spectra online.
- PDA detector allows one to choose a suitable wavelength for routine analysis.
- The absorbance ratio of two wavelengths can be calculated. When the ratio is constant, it gives confidence in the detection and quantification.
(iii) Refractive-Index HPLC Detector:
The Refractive Index (RI) detector for works on the principle of measuring the change in reflux index. The RI detectors are non-selective.
The RI detector used for HPLC comprises a glass chamber and is divided into two cells (or chambers). One chamber is filled with a mobile phase, and from the other chamber, a sample is passed through. The chamber from which the sample is flow-through is called a sample chamber or sample cell, and the other chamber is called a reference chamber or reference cell.

When the reference cell and the sample cell is filled with the mobile phase, the light beam passing through the chambers follows a straight path; however, when the sample cell is filled with the sample, the light beam gets bent because of the reflex index difference between the two solvents. This reflux index is measured to detect the presence of components in the sample.
The refractive index is sensitive to the temperature change; hence, the cell requires a good thermostatically controlled condition. Therefore, the detector cell is enclosed in a metal block. This metal block acts as a heat buffer.
Since the different liquids/ solvents have a different refractive index, the RI detectors in HPLC are unsuitable for gradient elution.
RI detectors have lower sensitivity, i.e., about 1000 times less sensitive than UV detectors. Because of this limitation, it is not a commonly used detector.
Advantages of RI detector compared to UV detectors for HPLC are:
- Able to detect most of the components.
- Suitable for the compounds that do not have UV absorption. Examples – sugar, alcohol, etc.
- Those solvents can be used having UV absorbance where such solvents can not be used for UV detectors.
- Intensity and analyte concentration are directly proportional.
Various setups are available for RI detectors in HPLC; however, the Deflection Refractometer and Interferometer are the two mostly used RI detectors.
(I) Deflection Refractometer for HPLC:

In the deflection type refractometer, the detector cell is divided into two parts. One part is filled with the mobile phase and another with the sample. When a light beam passes from the cell, the light gets deflected if the refractive indices differ in both the cells.
(II) Interferometer type refractometer for HPLC:

In an interferometer, the light from the source passes through the beam, which splits the light beam into two beams with identical intensity. One light passes through the sample cell, and another light is passed through the sample cell. Both beams are superimposed on a photodiode with the help of a splitter.
When the mobile phase passes through both the cells (sample and reference), the intensity of light differs with respect to the condition when the mobile phase passes in one cell and from the other cell sample passes.
(iv) Evaporative Light Scattering Detector (ELSD) HPLC Detector:

The working principle of the ELSD detector for HPLC is the nebulization of the sample solution. When the sample elutes from the column, the solvent or mobile phase evaporates, and only the sample remains in the droplet form because the solvent used in this system evaporates faster than the sample to be analyzed. Sample droplet remains in the gaseous stream as a dry particle and flows to the detector.
When a sample passes through the detector, it scatters the light beam. The quantum of scattered light is the measure of the concentration of analyte in the sample.
The evaporative light-scattering detector (ELSD) is nonselective in nature and used for nonvolatile analytes.
This method is more sensitive than the RI detector with a stable baseline as well as it can be used for gradient chromatography.
(v) Multi-Angle Light Scattering Detector (MALS) for HPLC:

Multi-Angle Light Scattering (MALS) detectors analyze the quantum of light scattered by the particulates in the sample relative to the angle of the light beam. For the complexes, macromolecules unfolded and strongly elongated proteins, multi-angle light scattering detectors are used to calculate Root Means Square Radius or Radius of Gyration. It reflects the mass distribution of an analyte compound surrounding its center of mass.
The Multi-Angle Light Scattering (MALS) HPLC detector typically has three and twenty cells arranged around the sample flow cell at various angles. This consists of a detector cell at 90°, and others are from 12° to 168°.
The Multi-Angle Light Scattering Detector Coupled with a concentration detector in HPLC directly measures the molar mass. The measurement is independent of the structure and shape of the molecule. This technique is useful to detect the molecular mass from 200 and above daltons.
This technique determines molecular weight without a calibration curve and is useful for compounds with very low detection limits.
(vi) HPLC-Mass Spectrometer (HPLC-MS) Detector
Using this HPLC-Mass Spectrometer, the elute gets detected based on its molecular weight. The application of HPLC-MS is to identify the compound structure and detect very low detection limits of elemental and molecular components.
HPLC-MS detector consists of three parts.
(i) Interface where the eluate enters the HPLC-MS detector to generate ions
(ii) Mass analyzer
(iii) Detector consists of an electron multiplier that determines the ion beam intensity
For the purpose of ionization, mainly two techniques are applied. (a) Atmospheric Pressure Chemical ionization (APCI) and (b) Atmospheric Pressure Electrospray Ionization (APESI), also called as Electrospray Ionization (ESI)
(a) Atmospheric Pressure Chemical ionization (APCI)

In this technique, ions are generated with the help of corona discharge, and molecule ions get generated. The advantage of this technique is that it can be used for small, medium, and nonpolar molecules. To facilitate detection, molecules should have some proton affinity and volatility.
HPLC-MS is not suitable for thermolabile components. This technique yield mass-sensitive signals.
(b) Atmospheric Pressure Electrospray Ionization (APESI) also called as Electrospray Ionization (ESI)

Using this technique, ‘coulomb explosion’ is created and it generates electrically charged ion droplets. This process generates ions, and it gives spectra showing molecule fragments.
In this technique, heating is not involved; hence, it can be used for thermolabile compounds and biopolymers.
This technique yields concentration-sensitive signals.
(vii) HPLC Conductivity Detector:
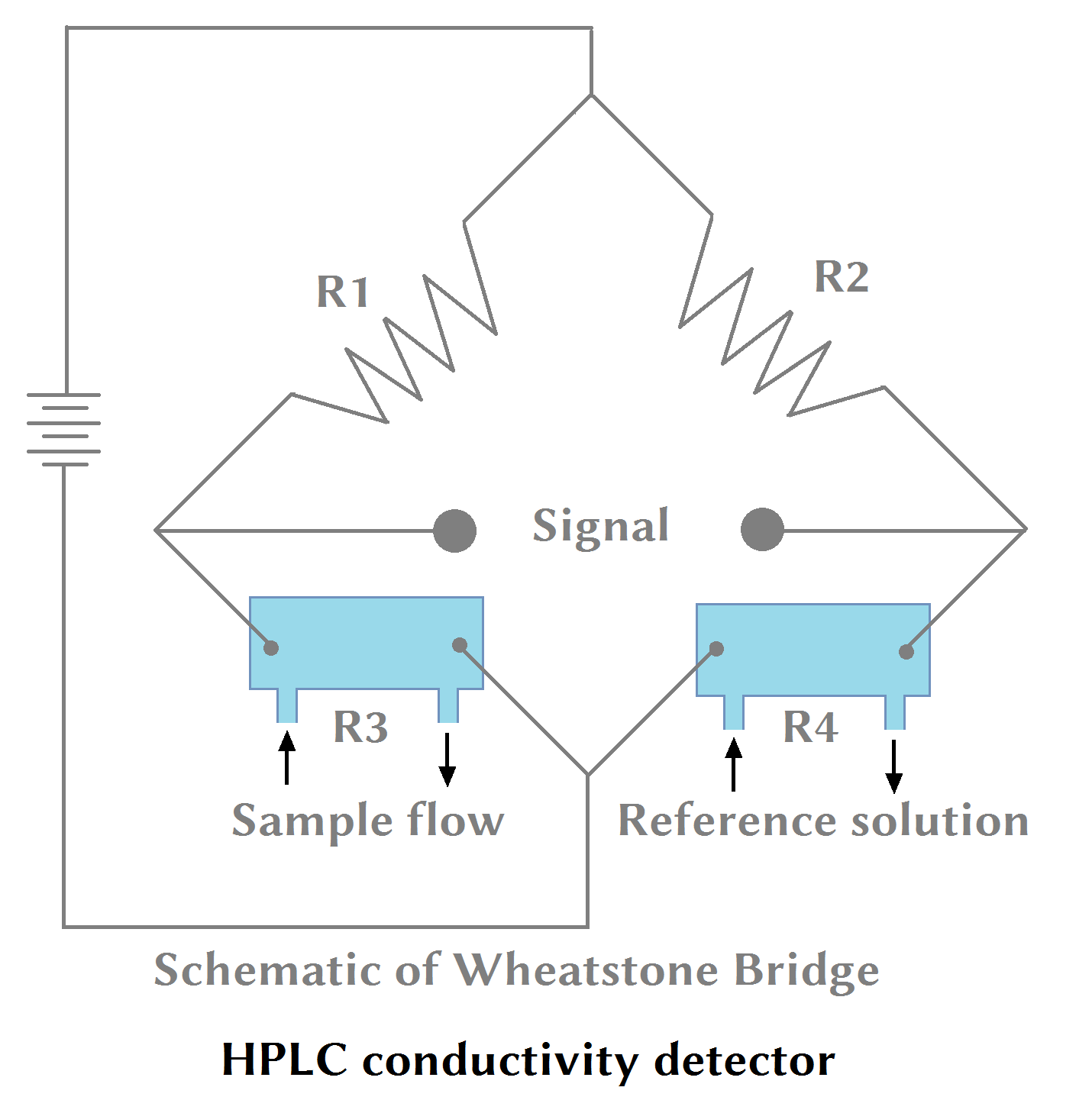
HPLC conductivity detector is used when the eluate conductivity is measurable. The conductivity/ resistance of the solution is directly proportional to the concentration of ions present in the solution under analysis.
The conductivity HPLC detector is temperature-sensitive; hence, it is important to maintain the temperature.
(viii) HPLC-Fluorescence Detector:

When some molecules absorb the light energy, it goes into an excited state, and when the electron returns to the ground state, light emission occurs. This phenomenon is called as fluorescence
The fluorescence HPLC detector technique is very sensitive for specific molecules.
HPLC-Fluorescence detector works on the principle of detection of emitted light, and concentration of analyte is directly proportional to the analyte concentration.
Natural products, clinical compounds, a good amount of pharmaceutical compounds, and petroleum products exhibit fluorescent behavior. For some compounds, to exhibit fluorescence behavior, it can be treated with suitable derivatives to get fluorescence.
To get efficient fluorescence excitation, excitation should be done at a lower wavelength that is more energetic in nature than the higher wavelength.
Xenon lamps provide wavelengths from 200 to 900 nm. Light emits as an outcome of fluorescence is in all directions; however, a photodetector for fluorescence detection is placed at right angles to minimize the interference. Sometimes, a UV detector is placed in a straight path to get combined fluorescence and UV absorbance results.
(ix) Chemiluminescence HPLC Detector:

Unlike fluorescence, chemiluminescence is the emission of specific wavelength light when electrons in the molecule return to a ground state from an excited state after absorbing external energy. The only difference is that instead of external wavelengths, the source of energy absorbed is a chemical reaction.
While using this technique for HPLC detection, derivatization is done when compounds elute from the column. Then, the solution for the derivatization process is added to the eluate using a delivery pump, which gets mixed with the elute.
Luminescence is generated after the process is quantified using the photomultiplier and photodiode.
(x) HPLC Optical Rotation Detector or Chiral Detector
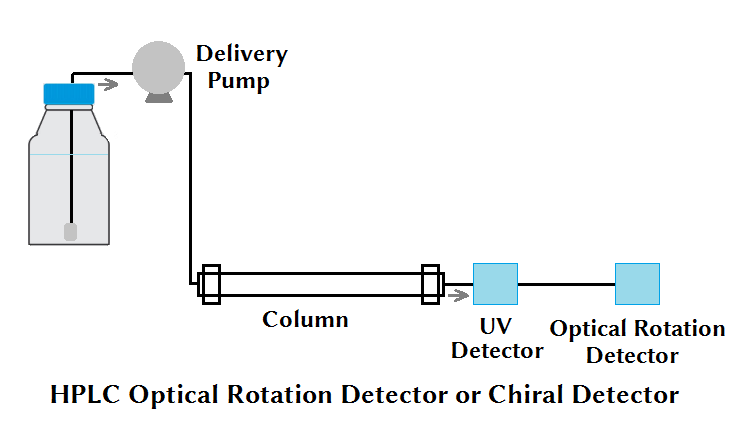
Detection isomer is a challenging task when using commonly used HPLC detectors such as UV, RI, etc. An HPLC Optical Rotation Detector or Chiral Detector serves the purpose to analyze the molecules that are optically active molecules based on optical activity (R- and L- type optical isomers).
The rotation of polarized light by optically active molecules can accurately determine the isomers with the help of the optical rotary power. The optically active molecule can provide information regarding its isomeric purity. This application is very helpful for quality control testing in the pharmaceutical and food industry.
Examples of optically active molecules are sugars and amino acids.
(xi) Electrochemical (Amperometric) Detector for HPLC

Once the compound gets eluted from the column, it enters into the electrochemical detector (ECD). When a compound enters into the detector, it gets oxidized or reduced.
When elute gets oxidized, it releases free electrons to the counter electrode, and when the analyte gets reduced, electrons are grabbed by the analyte from the counter electrode. Electrical current is directly proportional to the analyte concentration.
In this type of HPLC detector, the detection is based on polarography, amperometry, conductometry, and coulometry.
Aromatic amines, aromatic hydroxy compounds, phenothiazines, indoles, and mercaptans can be detected using oxidatively (using a positive potential). Reductive detection (negative potential) is not commonly used because dissolved oxygen and heavy metals cause issues. This method is used for nitrosamines and for pollutants.
(xii) HPLC Photoconductivity Detectors
In this detection technique, the analyte is parted in two directions post-column. One part is passed through the reference cell, and the other part is exposed to the UV light of 214 or 254 nm, whereby the analyte is photolyzed. The photolyzed fragments are detected with conductometric detection of ionic products. This technique is selective and sensitive to compounds containing halogen, nitroso, and sulfonamide groups.
(xiii) HPLC Infrared (IR) Detectors
All the organic compounds absorb IR waves at specific wavelengths. Fourier transform detector generally used as HPLC detector where the flow cell is made up of alkyl halides such as CaF2 or NaCl
While using the IR detector, the mobile phase should be carefully chosen that does not absorb IR waves at the required wavelength. Dichloromethane, Hexane, or acetonitrile are suitable mobile phases.
(xiv) Other HPLC Detectors
Apart From above, the following are also used depending on the applications.
- Laser-Induced Fluorescence Detector
- Radioactivity Detector
- HPLC-NMR Detector
(j) Data acquisition module
The data acquisition module consists of two components, viz. data acquisition, and data processing. The data acquisition module of HPLC acquires signals from the detector and converts analog signals to digital. The digital signal is further processed by the data processing unit and computed in numerical form and provides valuable information to analyze the data and provides a graphical representation of the signals called an HPLC chromatograph that is easy to read, understand, and interpret.
(k) Workstation to process acquired data
Workstation is the interface between a machine and a human. The workstation is used to program and command the HPLC, read and interpret the data and store the acquired data.
(l) Applications of High Performance Liquid Chromatography (HPLC)
The objective and application of HPLC are as follows:
Objective of HPLC is to separate the different compounds from solutions for the purpose of identification, production, quantitative analysis and purification of compounds.
Various applications of HPLC are as follows:
- Release testing of pharmaceutical compounds
- Identification of impurities in the medicinal products and pharmaceuticals
- Separation of impurities for characterization
- Clinical diagnosis such as:
- Thalassemia Screening by HPLC test before Pregnancy
- To quantify the concentration of amino acids during head injury of patients
- Analysis of drug concentration in the blood during the clinical studies and clinical trial
- Isolation of specific molecule from natural product and its purification
- Synthesis of active pharmaceutical ingredients by separation technique
- Production of biosimilars and antibiotics
- Enzymes detection
Frequently Asked Questions about HPLC
What is the HPLC principle?
Column chromatography has three main components. Stationary phase, Mobile Phase, and a sample from which components need to be separated.
What is a Stationary Phase: Unlike its name, it is the phase that does not move during the experimentation or analysis. Instead, it retains and reduces the flow of the components within the sample to be tested based on its affinity to the stationary phase, and the compound gets separated at different times.
What is Mobile Phase: It is a solvent or mixture of solvent that does move through the stationary phase. As it continuously flows through the stationary phase, it takes the compounds with it to separate the components of the sample.
A component that has a high affinity towards the mobile phase will elute quicker from the stationary phase. However, a component that has a high affinity with the stationary phase (column) will elute slower. The affinity of components means chemical attraction.
What are the modes of separation in HPLC?
1. Polarity
2. Molecular Size
3. Electrical Charge
What are the types of High Performance Liquid Chromatography (HPLC)?
2. Reverse Phase HPLC (RP-HPLC or RPC)
3. Size-exclusion HPLC (SEC) or Gel Permeation or Filtration Chromatography
4. Ion-Exchange HPLC
5. Bioaffinity chromatography or Affinity chromatography
What is the typical instrumentation setup of HPLC?
2. Mobile Phase or Solvent reservoir
3. Mobile phase transfer line
4. Frit (sinker frits in the mobile phase reservoirs)
5. Degassing system
6. High-pressure HPLC pump with manometer OR Solvent delivery system
7. Sample injector
8. Column
9. Column Oven or Column chamber with thermostat
10. Detector
11. Data acquisition module
12. Workstation to process acquired data
What is the HPLC Classification based on scale of operation?
2. Semi-preparative
3. Preparative
4. Process/ industrial production
What are the various types detectors used in HPLC instruments for analysis?
2. PDA Detectors (Diode Array Detector or Photo Array Diode Detector) HPLC detector
3. Refractive-Index HPLC Detector
4. Evaporative Light Scattering Detector (ELSD) HPLC Detector
5. Multi-Angle Light Scattering Detector (MALS) for HPLC
6. HPLC-Mass Spectrometer (HPLC-MS) Detector
7. HPLC Conductivity Detector
8. HPLC-Fluorescence Detector
9. Chemiluminescence HPLC Detector
10. HPLC Optical Rotation Detector or Chiral Detector
11. Electrochemical (Amperometric) Detector for HPLC
12. HPLC Photoconductivity Detectors
13. HPLC Infrared (IR) Detectors
14. Other HPLC Detectors – Laser-Induced Fluorescence Detector, Radioactivity Detector, HPLC-NMR Detector
To read about interview questions and answers on HPLC and troubleshooting click here
For more similar articles click here

