August 6, 2021: Pharmaceutical Interview Questions and Answers
#QbD #QualityByDesignSteps #QTPP #ControlStrategy
1. Explain typical steps for QbD steps.
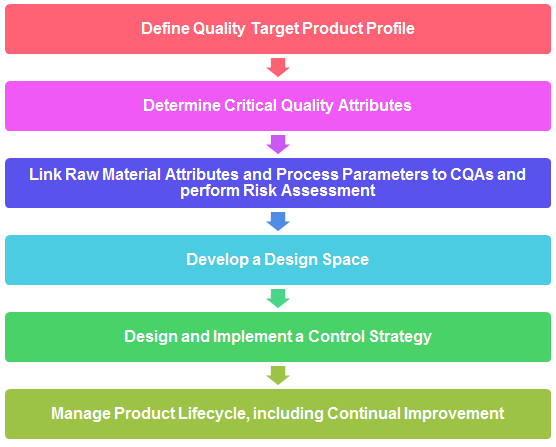
2. What is Quality Target Product Profile (QTPP)? Explain with examples.
The QTPP is defined by capturing all relevant quality requirements of the drug product to be developed.
It consists of following:
i. Dosage form and strength, route of administration, delivery systems, container and closure system etc.
ii. Drug substance quality attributes required for intended drug product, i.e. physical, chemical, and biological properties
iii. Drug product quality attributes required for dosage form, i.e. physical, chemical and microbiological attributes of drug products.
iv. Bioavailability attributes, i.e. dissolution requirement or other relevant characteristics. v. Excipient quality attributes, input material compatibility, stability, pharmacology, etc.
3. What is control strategy? What shall be included while defining control strategy?
• Raw material controls
• In-process and release specifications
• In-process controls
• Performance parameters
• Process parameter set points and ranges
• Process monitoring (data review, sampling, testing)
• Processing and hold times
• Process Analytical Technology (PAT)



