Thermal Cycling Study
What is a thermal cycling study?
A thermal cycling study is done to determine the impact of temperature excursion on the edge and outside of a product’s labeled storage conditions. The excursion is simulated below and above the product’s labeled storage conditions for multiple cycles, and the impact on the product quality will be evaluated because of thermal cycling.
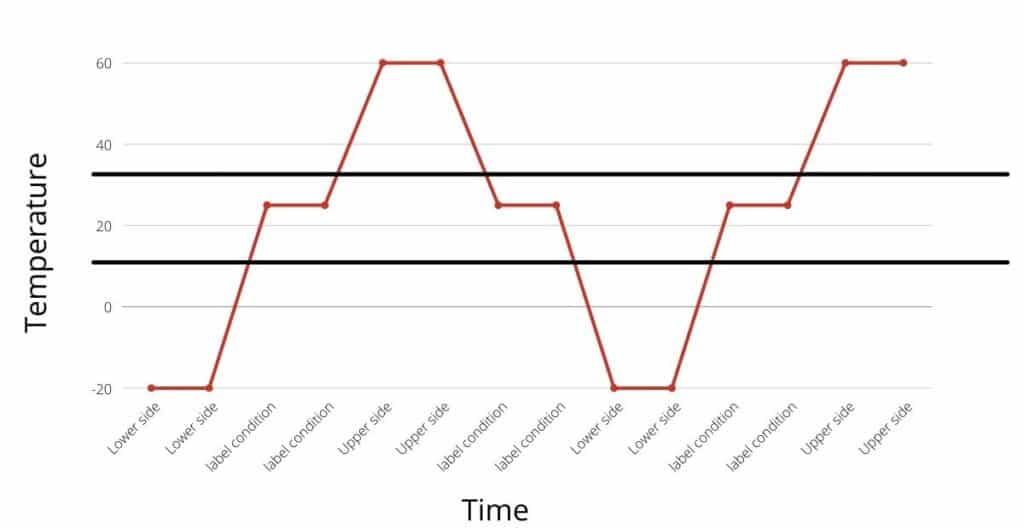
An example of thermal cycling is illustrated in the following diagrams.
How is thermal cycling study different from excursion studies?
A thermal cycling study is done by simulating upper and lower side excursion multiple times and assessing its impact on product quality. Whereas, in the temperature excursion study, the one-sided excursion is simulated without cycling.
What are the reference guidelines?
There is no direct reference in any of the regulatory guidelines regarding testing to determine the suitability of transport conditions for sensitive products; however, you can find general instructions in various guidance.
ICH Q1A and WHO guidance provide general direction to perform stress testing. The study’s objective is to evaluate the impact of stress conditions (temperature, humidity, oxidation, pH, and light) on drug products.
ICH Q1A and WHO also suggest that the data from accelerated stability studies can be used to evaluate the effect of short-term excursions higher or lower than the label storage conditions that may occur during the shipping of drug products.
The guidelines also direct for the drug substance to be stored in a freezer: “…testing on a single batch at an elevated temperature (e.g., 5°C ± 3°C or 25°C ± 2°C) for an appropriate time period should be conducted to address the effect of short term excursions outside the proposed label storage condition, e.g., during shipping or handling.”
Similarly, for the drug products to be stored in a freezer, “In the absence of an accelerated storage condition for drug products intended to be stored in a freezer, testing on a single batch at an elevated temperature (e.g., 5°C ± 3°C or 25°C ± 2°C) for an appropriate time period should be conducted to address the effect of short term excursions outside the proposed label storage condition.”
ICH Q5C directs for the stability of Biotechnological/ Biological Products that “Studies under stress conditions may be useful in determining whether accidental exposures to conditions other than those proposed (e.g., during transportation) are deleterious to the product.”
Work had done by Bishara and Seevers has now been included in the Parenteral Drug Association (PDA) documents. In this document, temperature excursions and temperature cycling conditions are proposed.

Why is thermal cycling study required?
Drug products are stored in the controlled environment in the facility, whereas when the products are transported in a commercial environment, it is a different scenario. Temperature conditions and excursions in the facility can be easily controlled; however, it may not be always possible during transportation. The impact on drug product due to unforeseen transport events could not be covered during the long-term and accelerated stability studies. Hence, the purpose of the thermal cycling study is to consider anticipated extreme challenges, including expected ambient temperature variation and shipment duration. In addition, the study will demonstrate the robustness of the product against usual excursions experienced during transportation.
How will it be helpful to justify excursions?
The drug product manufacturer’s responsibility is to ensure that the drug product reaches the patient without loss of therapeutic properties.
It is unpredictable that what types of excursion may occur during the transportation of pharmaceutical products, how long and how many times. For example, accidental temperature excursion may occur, which is beyond the pharmacopoeial acceptable excursion range. In such scenarios, the drug product would no longer be acceptable for patient consumption without sufficient scientific data.
Thermal cycling study results will help to determine product behavior and product quality impact when it experiences different environmental conditions during the transportation condition. In addition, the study data will help to understand the effect of accidental excursions on the drug product quality throughout the shelf life of the drug product.
How to design the thermal cycling study?
While designing the protocol for thermal cycling study, various factors should be considered such as product development data, Route of transportation, possible extreme conditions, and product strengths.

a. Support of product development data:
During the development of drug substances and drug products, R&D performs stress studies to determine the impact of various stress conditions on drug product, such as temperature, humidity, light, and oxygen. In this article, we will mainly discuss thermal stress. Suppose developmental studies show study product does not get degraded with extreme stress conditions. Those data may support accidental excursion during transportation, or these development data can support the design of thermal cycling study in terms of duration and extremes of stress condition. When development data shows the degradation of the product at specific stress conditions, there is no point in covering such situations during the study even though that could be expected excursion during transportation. In such a scenario, transportation facilities should be designed to maintain storage conditions so that such excursions would not occur.
b. Route of transportation:
The simplest way to design the study is to map the transportation routes from starting to recipient locations and identify the route’s seasonal temperature conditions and time required to reach from starting location to recipient locations. Typically two seasons are mapped, winter and summer. Consideration should be made for the day and night temperatures of the route. The external environmental temperature data can be collected using previous transportations or collected from historical meteorological data.
If one location is the Northern hemisphere and the other is the Southern hemisphere, the transportation route’s actual condition should be used. Such types of seasonal conditions are called summer-winter or winter-summer conditions. For example, a product is supplied to three different destinations. Temperature conditions should be mapped at the ambient environment to understand the potential excursions during transportation for respective destinations.
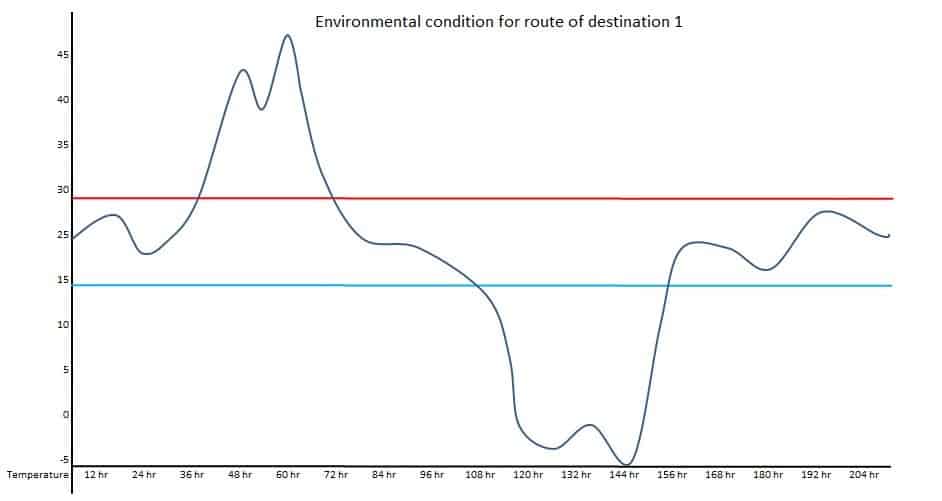

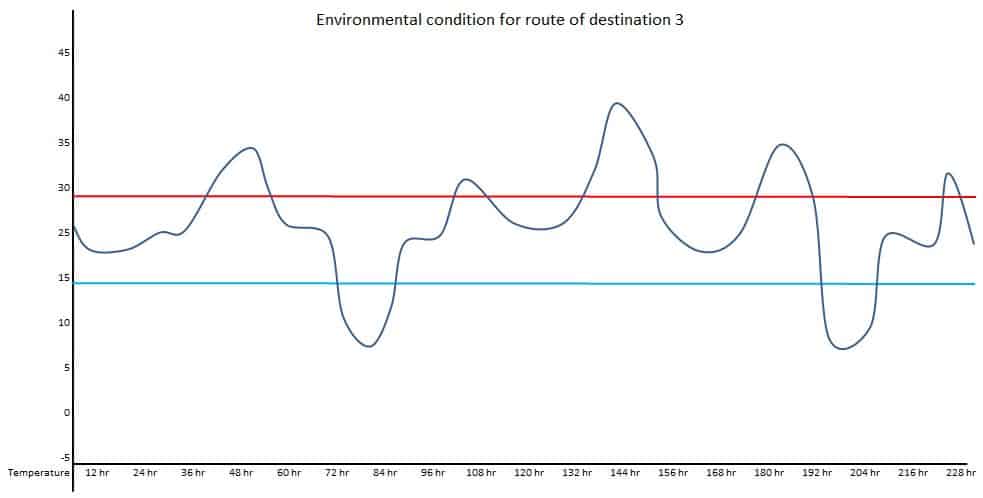
Data of above diagrams are summarized in following table.
Table: 1
| Excursion | Route 1 Duration | Route – 1 Temperature | Route – 2 Duration | Route – 2 Temperature | Route – 3 Duration | Route – 3 Temperature |
| 1 | 36 hr | Up to 47 Deg. C | 48 hr | Up to 5 Deg. C | 12 hr | Up to 35 Deg. C |
| 2 | 48 hr | Up to -5 Deg. C | 15 hr | Up to 33 Deg. C | 13 hr | Up to 7 Deg. C |
| 3 | 5 hr | Up to 31.5 Deg. C | 6 hr | Up to 31 Deg. C | ||
| 4 | 20 hr | Up to 40 Deg. C | ||||
| 5 | 12 hr | Up to 35 Deg. C | ||||
| 6 | 12 hr | Up to 7 Deg. C | ||||
| 7 | 5 hr | Up to 31 Deg. C |
Observed upper extreme temperature: 47 Deg. C
Observed lower extreme temperature: -5 Deg. C
Number of time upper side excursion during one run: 5 times
Number of time upper side excursion during one run: 2 times
Maximum total duration of upper side excursion: 55 hr
Maximum total duration of lower side excursion: 48 hr
Maximum duration of transportation: 228 hr
The above data can provide potential worst-case conditions during the transportation for the selected season. The study design for thermal cycling can be done based on the above data, such as total duration of the study, a number of excursion cycles, upper and lower extreme temperature, duration of each cycle, etc.
As per PDA Technical Report No. 39 (2007) suggest the following example for thermal cycling study for different storage conditions:
Table: 2
| Storage Condition | Testing Condition |
| Controlled Room Temperature 20 to 25°C | -20°C for 2 days followed by 40°C/75% RH for 2 days Repeat for a total of 3 cycles |
| Refrigerated Condition 2 to 8°C | 20°C for 2 days followed by 25°C/60% RH for 2 days Repeat for a total of 3 cycles |
| Freezer Condition -20 to -10°C | -20°C for 2 days followed by 5°C for 2 days Repeat for a total of 3 cycles |
The above approach can be modified based on collected data from the field study data presented in Table: 1 for the storage condition of Controlled Room Temperature, 20 to 25°C. [Opinion of the author of this article]
| Storage Condition | Testing Condition |
| Controlled Room Temperature 15 to 30°C | Step 1: Analyze the samples as per stability specification using stability indicating method Step 2: Expose the sample at -20°C for 2 days followed by 60°C/75% RH for 2 days Step 3: Analyze the samples as per stability specification using stability indicating method Step 4: Repeat the Step 2 and 3 two more times [total three times] Step 5: Load the above samples for long-term stability (25°C/60% RH) up to the shelf life. Total lower side excursion (-20°C) is 48 hr + 48 hr + 48 hr = 144 hrs Total upper side excursion (60°C/75% RH) is 48 hr + 48 hr + 48 hr = 144 hrs Long term stability study. The above duration is more than the total duration for upper and lower side excursions noted during the field study. Note: The study design can be done using the following two approaches: Approach 1: If initial analytical test results and test results after thermal cycling are analytically equivalent (up to Step 4), a long-term stability study (Step 5) can be skipped considering no significant change observed in the drug product after exposure to the stress conditions. Approach 2: Even though approach 1 complies, load the sample for long-term stability study condition to ensure that there is no impact of stress condition on the quality of the product up to the end of shelf life (most preferred approach). |
c. Consideration of extremes:
There are natural temperature ranges that could be defined. It will be very unusual to have temperatures below −100°C or over 100°C in a natural environment. In Antarctica, temperature minima have been measured at −89°C. Temperature maxima have been measured at +58°C in Libya and in Death Valley. But even if a product is stored in a closed environment under the sun, it will not be heated over 100°C.
d. Consideration of product and strength selection:
The bracketing approach with higher and lower strength can be considered as one of the approaches. Product strengths to be bracketed can be defined using the Quality Risk Management principle.
Conclusion:
Systemically designed thermal cycling study will provide valuable insight about product robustness under stress condition and accidental exposure of product with high or low temperature can be scientifically justified. Based on the study performed, Product-Specific Transportation Control Strategy Document can be prepared for to determine the Effect of Temperature Excursions as follows:
| Temperature Range | Time |
| <-20°C | Do Not Use |
| -20 to 15°C | 2 days |
| 15 to 30°C | Until Expiry |
| 30 to 60°C | 2 days |
| >60°C | Do Not Use |


