
Change Control Program or Change Management System
Change Control Program is the most important pillar of the Quality Management System (QMS). The system is followed by various industries such as pharmaceuticals, biopharmaceuticals, biotechnology, Active Pharmaceutical Ingredient manufacturers, and organizations following QMS as per ISO 9001. Process steps for change management remain similar irrespective of industry and organizations. However, it requires customization depending on the individual organization’s needs, types of business model, and regulatory requirements. The change control program is also called a Change Management System.
The examples covered in this article are of pharmaceutical industry.
1. Reference guidelines for Change Control Program or Change Management System
Change control program is an essential element of pharmaceutical quality systems and referred in various guidance and regulatory documents. Following are few references of guidance and regulations.
EudraLex:

EudraLex:

Code of Federal Regulations (CFR): 21 CFR, 211.100 and 21 CFR, 211.160


PI 006-3 Recommendation on Validation Master Plan

PI 002-3 Quality System Requirements For Pharmaceutical Inspectorates
Quality assurance of pharmaceuticals, Volume 2, 2nd updated edition Good manufacturing practices and inspection, WHO”

2. Types of changes
Changes in pharmaceutical industry can be broadly classified as product specific changes and general changes. These two groups can be further expanded as follows with examples of changes:
- Product specific changes:
- Change in batch manufacturing record and batch packaging record:
- Batch size change
- Change in formula
- Change, addition or removal of equipment
- Change in manufacturing process
- Tightening or relaxation in manufacturing in-process parameters
- Change in document template
- Inclusion of additional instruction/ simplification or editorial changes without changing actual process
- Artwork change
- Change in pack profile or configuration
- Change in holding time of in-process materials
- Change in batch size
- Change in specification and method of analysis:
- Revision of specification limits
- Change in analytical procedure
- Pharmacopoeial changes
- Tightening or relaxation in manufacturing specification
- Addition or deletion of test
- Change in document template
- Inclusion of additional instruction/ simplification or editorial changes without changing actual method of analysis and specification limit
- Other product specific changes
- New-product introduction
- Change in vendor of product specific starting materials
- Change in starting material manufacturing process
- Change in customer of product
- Change in storage condition based on destination market
- Change in master formula records
- Addition or deletion of stability study condition
- Extension or reduction of product shelf life
- Change in batch manufacturing record and batch packaging record:
- General changes:
-
- Change on Standard Operating Procedure (SOP)
- Facility change
- Equipment change
- Modification of equipment
- Upgrade equipment or system software
- Permanent change in working shift timing
- Change in layouts and drawings
- Modification of facility
- Heating Ventilation and Air Conditioning (HVAC) system modification
- Revision of documents, such as, Site Master File, Validation Master Plan, Quality Manual, Protocols, or any other GMP documents
-
3. Rating or classification of changes
Change controls required to be further classified based on nature and criticality of impact on system, facility, equipment/ instrument, material, product, procedure or process. Depending on the criticality, changes can be classified as Major Changes or Minor Changes.
-
- Major change:
Major changes are those changes that have a direct impact or potential impact on the product quality, safety, strength, identity, efficacy, and purity of a product, validation status of the process, equipment, utility, facility or GMP compliance, procedures, systems or regulatory filings.
-
- Minor Change:
Minor changes are those changes that do not have a direct impact or potential to have a direct impact on the product quality, identity, safety, strength, purity, and efficacy of a product or validation status of the process, equipment, utility, facility or GMP compliance/ procedures, and systems or regulatory filings.
| Table: 1 – Classification of change control | ||
|---|---|---|
| Major changes | Minor changes | Changes do not require change control |
|
|
|
4. Category of changes
A change may be permanent or temporary.
-
- Permanent changes:
Permanent changes mean the changes that remain in effect till the next required revision or modifications.
-
- Temporary change controls:
A change that is implemented for a specific predetermined period. Temporary change controls are also called planned deviation by some of the organizations.
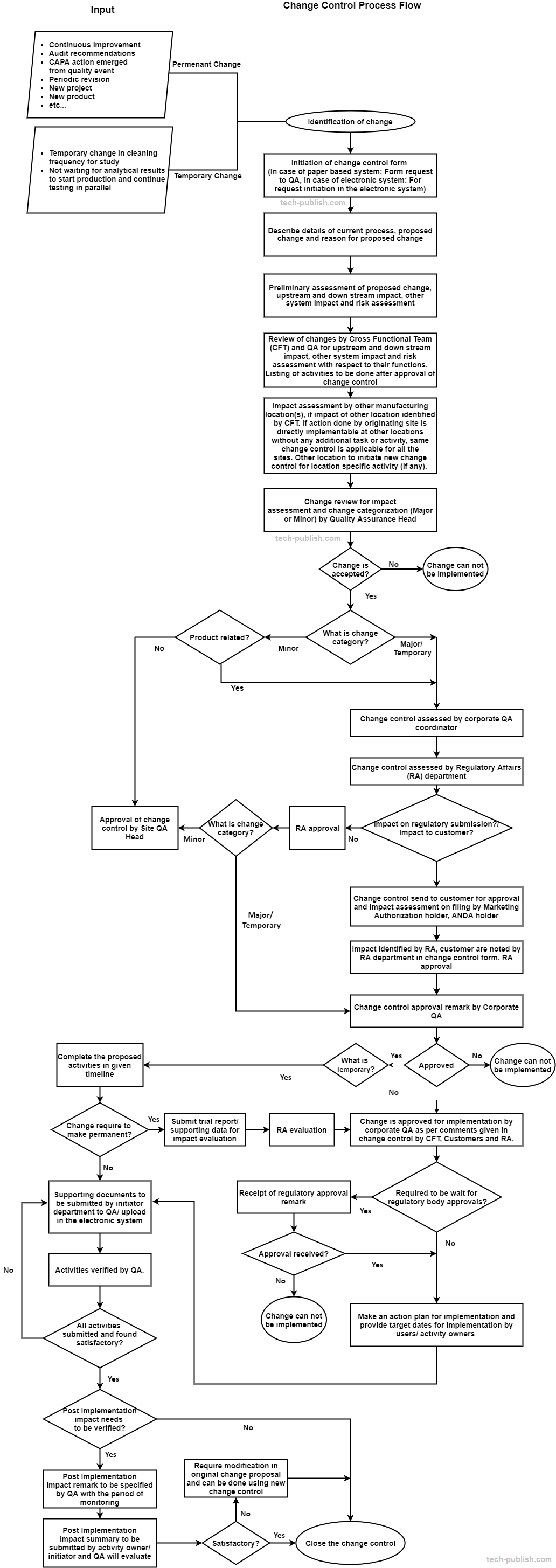
5. Change management process flow
Diagram: 1 – Typical change management process flow
6. Impact and risk assessment
Any change in the system or process, there is an impact. The impact could be favorable, which is expected as an outcome of the change proposal. However, there could be an impact on upstream and downstream processes or related systems because of the change. The effect could be for the initiator department, other cross-functional departments, other locations, regulatory filings, validation status of product, site approval status. The impact needs to be assessed before the change proposal is approved. There is a crucial role of initiator, cross-functional team, regulatory affairs department, and Quality Assurance department. If the change requires a mitigation plan, it shall be documented as a part of the change control assessment and actionable while implementing the change.
The impact can be assessed under the following headings but not limited to each change. These are few examples; however, it could be different depending on organizational structure, regulatory markets, and other quality system elements.
| Table: 2 – Parameters to be considered for impact and risk assessment for change control evaluation | ||
|---|---|---|
| Sr. no | Impact of change to be evaluated for | Sub sections |
| 1 | Facility approvals |
|
| 2 | Buildings and facilities |
|
| 3 | Equipment/ instrument |
|
| 4 | Components and drug product containers and closures |
|
| 5 | Production and process controls |
|
| 6 | Packaging and labeling control |
|
| 7 | Holding and distribution |
|
| 8 | Laboratory controls |
|
| 9 | Records and reports |
|
| 10 | Electronic system |
|
| 11 | Aseptic processing |
|
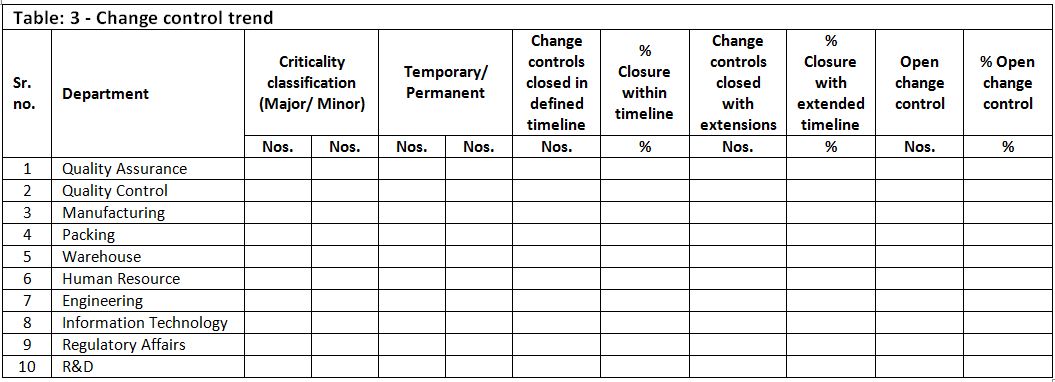
7. Trending of change controls
Trending of changes in the facility is very important to monitor and control the changes. Changes which were rejected, canceled, major changes, open changes etc. are required to be closely monitored and trended. During regulatory inspections, primary focus are major changes, facility related changes, rejected changes etc.
Following is an example template which can be used for trend analysis.
8. Post implementation review of change controls
Changes such as major changes, new procedures, upgraded procedures, procedure implemented in response to deviation or complaint CAPAs and compliance require post-implementation review that after implementation of the changes, it is giving intended outcome. Measurement criteria can be qualitative, qualitative, or a mix of both. For example, a reduction in the number of complaints after implementation of new process, that could have implemented as an outcome of a complaint investigation. Reduction in the number of human errors and reduction in documentation errors can also be considered a measure of post-implementation review.

